1. Introduction
The European Union is an international leader in the bioeconomy sector: Germany, France and Italy are the greatest global producers of crops, meat, fish and processed food, which determine the production of large amounts of residual organic streams, mainly livestock manure. The total European production of livestock effluents accounts for 1.4 billion tonnes per year, with France, Germany and Italy producing 276, 197 and 103 million tonnes per year, respectively [
1]. The production of agricultural residues derived from crop cultivation accounted for 21 million tonnes in 2020 in the European Union [
2]. These streams, collectively named agro-waste, are a rich untapped source of carbon and nutrients and should not be disposed of massively but rather properly valorized within the circular economy concept with the potential to support human well-being and saving resources [
3].
Anaerobic Digestion (AD) is a robust technology for the management and valorization of agro-waste in the rural context: in fact, AD enables the bioconversion of the organic matter present in manure, residual crops, and other residual organic streams of the food processing chain while recovering biogas for power or biomethane production [
4,
5] and a digestate containing considerable quantities of valuable nutrients [
6].
Because of its benefits and the incentives schemes applied in different Countries, AD is largely diffused in Europe [
3]: more than 18,000 plants with an installed capacity of 8000 MWel are globally under operation in Europe according to the European Biogas Association [
7]. In 2021, the EU produced 3 billion m
3 of biomethane [
8]. However, the EU production should increase to 35 billion m
3 by 2030, to be able to comply to the new EU communication COM/2022/108, implementing the “RE. PowerEU” plan.
As reported above, besides biogas, AD plants generate also digestate, a sort of renewable fertilizer [
3,
6]. The use of digestate provides macro (nitrogen, phosphorus, potassium, etc.) and micro (cobalt, selenium, etc.) nutrients to the soil. Moreover, it determines the supplementation of stable carbon to the fields, thus increasing the carbon sink capability of soils [
9].
In the last 20 years, the number of AD plants has increased considerably in Western and Central Europe because of favorable incentive schemes dedicated to the supporting of power and biomethane generation [
10]. However, the incentive schemes for power generation in Germany, Italy and Austria are rapidly coming to an end, therefore the use and exploitation of rural anaerobic digesters should be reconsidered.
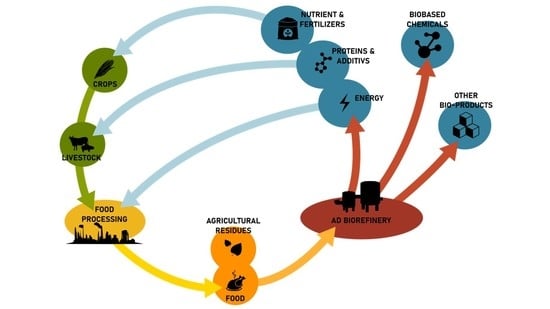
In perspective, an obvious upgrade of this technology is the transformation of anaerobic digesters into a multi-feedstock, multipurpose and multiproduct biorefinery able to valorize agro-waste into high added value biobased products such as nutrients and chemicals, thus creating social and economic benefits at local level [
11,
12,
13].
In recent years, several studies showed the possibility to generate biofuels such as hydrogen and methane, biobased chemicals such as polyhydroxyalkanoates, produce microalgae, bacteria or yeast, convert CO2 and recover nutrients from organic wastes.
It is therefore possible to imagine anaerobic digestion at the center of a future biorefinery approach where agro-wastes and food processing waste are converted into high added value biobased products other than biofuels. This new bioeconomy approach is crucial for the rural renaissance of Europe.
In this framework, the Horizon Europe project AgriLoop will study the upcycling of agro- and food-processing waste and byproducts to high value biobased products. A pilot scale platform biorefinery will be operated where cow manure, crops silage and other agro-waste will be transformed into biofuels, carboxylic acids, and polyesters, while nutrients will be recovered in concentrated forms.
In this paper, we will provide evidence of this global approach, here implemented in a pilot scale biorefinery able to convert agro-waste into valuable products working in a real environment. For the purpose of this study, agricultural wastes, by-products and co-products are defined as plant or animal residues that are not (or not further processed into) food or feed. This large amount of undervalued biomass is further called agro-waste as it is intended to be fully integrated as resource in optimised cascading biorefinery and therefore revoked as waste, to become multiple levers of the transition away from a fossil-based carbon-intensive economy.
2. Materials and Methods
The general approach proposed in this study envisages that livestock effluents, crop silage and other agro-waste are transformed into biofuels and biobased products such as volatile fatty acids (VFAs) and polyhydroxyalkanoates (PHAs), while nutrients and microbial proteins are recovered. All these activities will be carried out treating real substrates and in a real environment: the pilot scale platform is placed in “La Torre” farm, Isola della Scala, Verona, in the northeast of Italy.
This biorefinery platform will allow for the treatment of different types of feedstocks and their bioconversion into biobased products.
2.1. Pilot Plant Description
The biorefinery plant is an upgrade and modification of the one presented by Righetti and colleagues [
13], first developed in the framework of the project NoAW, No Agro-Waste-Innovative approaches to turn agricultural waste into ecological and economic assets (
http://noaw2020.eu/), accessed on 20 December 2022, and it can be divided into the following main areas:
agro-waste pre-treatment and preparation;
acidogenic fermentation where volatile fatty acid production and hydrogen are produced;
mixed microbial biomass selection and PHA storing;
PHA extraction and purification;
anaerobic digestion of the solid part of the fermentation effluent;
nutrient recovery;
microbial protein production.
The overall approach of the biorefinery and the obtainable products are schematically shown in
Figure 1.
The 2 m3 storage tank for feedstock mixing and preparation is equipped with a weighing system for the control of the amount of material fed into the system. The tank has a mixer to homogenize the substrates. Mixed material is then ground by means of a special centrifuge pump model TM451 (Vidotto Dissipatori, Eraclea, Italy, Italy) and pumped to the anaerobic acidogenic reactor. The fermentation unit is a 5 m3 unit with an adjustable volume. The fermenter is temperature controlled with warm water and can operate both in mesophilic and thermophilic conditions.
The operational conditions applied to the reactor are shown in
Table 1.
The fermentation process operates in mesophilic conditions with an average organic loading rate in the range of 15–25 kgCOD/m3 d, a desired OLR of approximately 18 kgCOD/m3 d and a hydraulic retention time of 3 to 5 days to improve the conversion of particulate COD into short chain VFAs to be fed to the PHA bioreactor. The operational pH of the anaerobic fermenter is determined by the characteristics of fed substrates and it is typically around 5.5. Operational conditions such as OLR can be set in order to tune the abundance of different short chain VFAs in the liquid phase.
The fermenter effluent is treated in a horizontal screw press unit (Sepcom, WAM Group, Modena, Italy) for the solid/liquid separation. The screw press treatment capacity is 5 m3/h.
The liquid fraction obtained through solid/liquid separation is then pre-filtered at 500 micron and then micro-filtered in a rotating ceramic unit. The permeate rich in short chain VFAs is then sent to the mixed microbial biomass selection unit.
The first bioreactor is a 2 m3 volume system for the selection of PHA-accumulating microorganism, while the second reactor, 1 m3 in volume, is dedicated to the accumulation of PHAs.
Both bioreactors can operate in batch or continuous mode.
The sludge rich in PHAs produced in the second bioreactor, after settling and concentration, is quenched with sulfuric acid and sent to a chemical extraction unit.
The separated solid fraction obtained from the screw press is sent to a mesophilic anaerobic digester designed to operate at an optimal organic loading rate of 4 kgVS/m
3 d and with a hydraulic retention time of approximately 30 days. The anaerobic digester has a working volume of 1 m
3. The operational pH of the anaerobic digester is typically self-buffered around 8.
Table 1 reports the expected ranges of operation for both OLR and HRT.
The anaerobic digestion effluent undergoes to solid/liquid separation and the liquid stream is sent to a screening and filtration unit with mesh of 250 µm and then to a double stage filtration unit where ultra-filtration is followed by reverse osmosis for nutrient recovery and concentration. The permeate (clean water) is used to dilute the treated feedstock in the initial storage tank. The ultra-filtration unit has a ceramic disc filter with a porosity of 0.22 µm, with a maximum flow of 10 L/h. The reverse osmosis unit has a membrane porosity of less than 1 nm, with a maximum flow of 200 L/h.
2.2. Analysis
In order to carry out the mass balances for the system and for each operation unit, the feedstocks and the effluents of the reactors were monitored once or more times per week through the analysis of total and volatile solids (TS and TVS), chemical oxygen demand (COD), Total Kjeldahl Nitrogen (TKN) and total phosphorus (TP) concentrations. The process stability parameters for the anaerobic fermenter and digester, namely pH, VFAs content and speciation, total and partial alkalinity and ammonia (NH
3-N) were regularly checked daily. All the analyses, except for the VFAs, were carried out in accordance with the standard methods for water and wastewater analysis [
14].
The biogas flowrate was measured by a volumetric counter (Ritter Gmbh, Schwabmünchen, Germany), while biogas composition in terms of CH4 and CO2 concentrations was measured using a portable gas monitor (GAS Counter BIOGAS 5000), equipped with a hydraulic guard to remove H2S. Hydrogen content was determined by a gas chromatograph (GC Agilent Technology 6890 N) equipped with the column HP-PLOT MOLESIEVE, 30 × 0.53 mm ID × 25 μm film, using a thermal conductivity detector and an argon as gas carrier.
2.3. Mass Balance Equation and Yields of the Pilot Plant
The mass balance of the pilot scale plant is based on the chemical characteristics of the influent and effluent streams and the relative flowrates. The general equation for mass balances for a given chemical compound considers influent and effluent mass, generated and consumed mass and a term for accumulation according to the general formula:
The general equation can be then applied considering flowrate and concentration for any chemical compound according to the following equation:
where
Qin is influent flowrate, m3 per day;
Cin is influent concentration of a given compound, kg per m3;
Cout is effluent concentration of a given compound, kg per m3;
Qout is effluent flowrate, m3 per day;
C is compound concentration in the bioreactor, kg per m3;
V is volume of the bioreactor, m3;
Rp is specific production rate in the system, kg per m3 per day;
Rc is specific consumption rate in the system, kg per m3 per day.
In steady state conditions, the concentration of a given component, C, is constant and Equation (2) equals zero.
Yields for a given product are determined considering the quantity of product per time unit, typically per day, over the quantity of substrate entering the system at the same time.
The mass balance and yields of the system discussed below are determined according to the description reported here.
3. Expected Results and Discussion
As described above, existing AD plants can be converted into multipurpose biorefineries able to produce a portfolio of different biobased products.
In this study, we will consider the conversion of a mix of cow manure, straw and residual crops into different bioproducts:
- -
hydrogen and methane,
- -
short chain VFAs,
- -
PHAs,
- -
nutrients,
- -
microbial proteins.
The scheme of the general approach is shown in
Figure 1.
Specific details for the different biobased products are illustrated and discussed below.
3.1. Biofuels: Hydrogen and Methane Production
Hydrogen and methane have gained particular interest in the scientific community in recent years due to the need of decarbonisation to fight climate change and to the increasing price of natural gas. This has led to the further development and implementation of the two-stage anaerobic digestion technology, a biological process which allows for the simultaneous production of hydrogen and methane from organic residues. The two anaerobic reactors treating agro-waste are dedicated to dark fermentation and methanogenesis, respectively. The first step consists in hydrogen and carbon dioxide production by the acidogenic microorganisms, through the hydrolysis of complex organic compounds into soluble macro-molecules and then into short chain VFAs. During the methanogenic step, in the second reactor, VFAs are degraded to carbon dioxide and methane, while part of the carbon dioxide is bio-converted to methane through its reaction with hydrogen [
15]. The resulting gaseous blend can be upgraded to eliminate undesired compounds such as hydrogen sulphide, ammonia and carbon dioxide [
16] in order to obtain the hydrogen–methane blend bio-hythane.
In the AgriLoop project, we will operate the two reactors as described in
Table 1 and we expect yields of approximately 20 L H
2 and 180 L CH
4 per kgVS treated. Besides the gaseous effluents, the process produces digestate, a mix of solid and liquid parts.
3.2. Volatile Fatty Acids Production
VFAs are short chain carboxylic acids from acetate (C1) to caproate (C6), usually derived from fossil sources. The biological production of VFAs is receiving great attention to favour the transition from a linear to a circular economy model. Through anaerobic fermentation, agro-waste can be converted into valuable VFAs with high economic value, ranging from 800 to 2500 €/tonne [
17]. In our project, agro-waste will be converted into VFAs for the synthesis of PHAs and single cell proteins (SCP, see
Section 3.5).
The VFAs yields depend on the chemical and physical nature of the organic substrates: in this case, the mixed material will be composed of both hard-to-degrade organic material (straw) and easily biodegradable cellulose and hemi-cellulose, therefore expected yields are in the range of 0.2–0.3 gVFA/gVS. These values are in line with literature data: agro-food wastes rich in simplest compounds, for example, winery waste such as lees, having high content of glucose, fructose and ethanol, have typical VFAs yields of 0.5–0.7 gVFA/gCOD; olive pomace, rich in lipids and recalcitrant lignocellulosic compounds, have lower yields [
18]. In order to increase the process yield, lignocellulosic materials represented by rice, corn, wheat straws and by-products from energy crops are usually pre-treated under steam explosion to favour the degradation of the long and complex carbon chains into smaller organic fractions, which can be easily converted by the mixed microbial culture [
19], increasing the VFAs yield to 0.45–0.55 gVFA/gCOD [
20].
In this specific study, chemical pre-treatments to increase bioconversion of recalcitrant organic matter will be applied; in particular, the use of soda will be tested.
In particular, crop residues and manure with straw will be added with soda at 36% to match up a final concentration in the pre-treatment vessel of 1% and digested overnight at environment temperature.
In terms of concentration, VFAs in the fermentation broth are usually in the range of 15–40 g/L [
21] depending on both bioconversion capability and dilution. To be exploitable for industrial purpose, VFAs need to be separated from the other organic compounds and concentrated in order to have a minimal concentration level of 100 g/L [
22].
Several techniques such as adsorption on solid matrix [
23], distillation and evaporation [
22], extraction by solvent [
24]
, electrodialysis [
25] and pressure-driven membrane processes [
26] have been implemented for VFAs recovery. Traditional recovery techniques by solvents and distillation are known to be high in cost and energy, but are still largely applied in order to separate VFA from fermentation medium. It was demonstrated that the maximum VFA recovery by this technique falls in the range of 61–98% [
27]. Filtration techniques, such as nanofiltration and reverse osmosis, are becoming attractive alternatives to traditional recovery techniques, being capable of recovering over 90% of the VFAs content from the fermentation medium [
28].
VFA recovery has other advantages if applied directly on the liquid phase of the fermenter: it can enhance anaerobic fermentation because (i) it shifts the balance toward VFAs biosynthesis, (ii) it alleviates product-induced inhibition, and (iii) it maintains stable alkalinity levels, allowing further waste degradation [
29].
In order to obtain a functional cascade of integrated membrane separation processes that allow the recovery, purification and optimal concentration of VFAs from waste-derived AD effluents, it is essential to have pre-treatment processes able to deeply remove suspended solids and colloids. To ensure the long-term application of a membrane filtration system, it is necessary to take into account the factors that influence filtration operations, such as pH and solids content (TS and TVS) [
28]. The solids content is the limiting factor for the application of specific membranes, since it can cause damage to the membrane itself. Consequently, pre-treatments such as solid/liquid separations with a centrifuge or vibrating screen must be applied [
17].
In the AgriLoop project, because of the bio-recalcitrant nature of treated agro-waste, mainly due to straw, expected yields are low, in the range of 0.1–0.2 kgVFA per kg of VS fed to the anaerobic fermenter, while the fermentation broth will show VFA concentrations in the range of 10–15 g/L. Several recovery techniques will be tested at lab scale with a preference for membrane contactors.
3.3. Polyhydroxyalkanoates Production
Polyhydroxyalkanoates are a family of thermoplastic polyesters of hydroxyacid (HA) monomers connected by an ester bond [
18]. They can be produced from different renewable sources by bacterial fermentation, as a form of intracellular carbon and energy storage. The process of PHA production by mixed microbial culture applied in this biorefinery platform is conducted in three independent stages [
30]. In the first step, the complex organic substrate is fermented to obtain a stream rich in VFAs; the second step is based on the natural principles of selection and competition of PHA-accumulating microorganisms against microorganisms that are unable to accumulate PHAs, by applying transient conditions in sequential batch reactors (SBR) [
30]. It has been shown that conditions of external substrate excess (feast) and limitation (famine) select microbial populations with an increased capacity to store PHAs [
31]. Finally, in the third phase, when the cells reach maximum PHAs content, they are harvested and sent to downstream extraction processes [
18]. The downstream processes for extracting the polymer from the cells are among the most important factors affecting the overall cost of PHAs production, and they represent the bottleneck of the whole process. So far, the most studied methods for the recovery of PHAs can be grouped into two categories: solvent extraction and digestion of the non-polymeric material. Solvent extraction is the predominant method for PHAs extraction, used when high polymer purity is desired [
32]. It typically involves soaking the PHA-containing biomass in an appropriate solvent or solvent mixture to dissolve the granules, followed by the addition of a precipitating agent to recover the polymers in crystalline form [
33]. In the dissolution step, chlorinated solvents are typically used, e.g., chloroform and dichloromethane. For the precipitation step, methanol and ethanol are used [
33]. Solvent extraction in large-scale applications is generally not an environmentally friendly method due to the harmful characteristics of the most used solvents, especially chlorinated solvents such as chloroform, and the massive amounts of antisolvent used (approximately 10 volumes per volume of PHAs solution) in order to precipitate the polymer. However, PHA-producing microorganisms can accumulate these polymers in quantities of up to 70% g PHA/gVSS [
18], therefore it is possible to remove the small fraction of non-PHA cellular mass (NPCM) surrounding the PHA rather than extracting the PHA by solubilizing it in a suitable solvent. For this reason, several methods have been developed to release the PHAs granules by solubilizing the surrounding NPCM. Various chemical compounds can be used: sodium hypochlorite, surfactants, acid and alkaline compounds. All these chemicals can be used in the aqueous phase, thus avoiding the energy consumption required to dry the biomass when solvent extraction is applied [
33].
Although the mass balance indicates higher productions, the expected final PHA productions for this pilot scale biorefinery process can reach a maximum of 2 kg PHA per day with a yield estimated in 0.01 kgPHA per kgVS in the feedstock because of the complexity of the biomass treated in this specific context, mainly due to straw, and because of the set-up of the extraction and purification phase.
3.4. Nutrient Recovery
The main by-product of the AD process is represented by a solid-liquid mixture, the digestate, which contains the stabilized organic matter not converted into biogas and considerable amounts of macro- and micro-nutrients. Digestate is now receiving great attention from the agricultural community because it is rich in nitrogen and phosphorous, and because of the high costs of fossil fertilizers. For example, when livestock effluents are treated, TKN concentration can reach values in the range of 2.5–9 g per kg of fresh matter with an ammonium content exceeding 50–60%
w/
w [
17]. Agricultural digestate have also a good content of phosphorous, typically in the range of 0.5–1.5 g per kg fresh matter, a small part of which is in the soluble form.
There are currently several technological options for digestate treatment and nutrients recovery available on the market. All the options require a common step of solid/liquid separation of the agricultural digestate. These two phases, solid and liquid, can be then further processed to obtain concentrated nutrient streams minimizing the transport costs [
16,
34].
The most developed technologies at industrial scales for nitrogen and phosphorus recovery are ammonia stripping and struvite precipitation, respectively, which allow both an average removal and recovery yields of 90–95%. However, they have very high environmental and energetical costs [
17]. For this reason, new technologies are emerging to reduce the use of chemicals, such as pressure-driven membranes, ultrafiltration and reverse osmosis (RO).
Membrane processes are physical-based technologies, where the agricultural digestate is treated under sequential solid/liquid separations. The solid fraction from each stage is called concentrate or retentate, while the liquid phase is named permeate. Usually, the first separation stage consists in a preliminary removal of the coarse solids by means of a centrifuge or a vibrating screen. Then, the liquid is treated in several steps: (i) microfiltration (MF), having membrane with pore size >0.1 µm under a trans-membrane pressure of 0.1–3 bar, (ii) ultrafiltration (UF, pore size >0.001 µm, pressure 2–10 bar) and a (iii) RO (pore size <1 nm, pressure 10–100 bar), to obtain a nutrient-rich retentate and water as permeate. In this way, it is possible to obtain a fertilizer rich in N and P (8.2–12.0 kg/tonne TN; 5.6–10.4 kg/tonne P
2O
5) [
19,
20].
In this study, we will treat the liquid fraction of digestate obtained after solid/liquid separation via a screw press in a train of operation consisting of filtering/sieving, microfiltration and reverse osmosis to obtain a retentate characterized by relatively high concentrations of nitrogen, potassium and phosphorus, as well as other important minerals such as iron, magnesium and sulfur, with the characteristics of distillation vinasse which can be used as soil amendment. Expected typical concentrations for nitrogen are in the range of 7–10 gN/L [
35] while phosphorus is mainly recovered in the form of fine microfiltered sludge before the RO unit.
3.5. Proteins Production
Due to the growing human population, expected to reach 10 billion by 2050 [
36], proteins are a sought-after resource not only as a source of food or animal feed, but also as the starting material for other high-value products such as protein hydrolysates, biostimulants for agriculture, wood adhesives, flocculants, surfactants and protein-based plastics [
37]. Animal- and vegetal-based proteins are at present mainly produced through low-efficiency processes with a high environmental impact in terms of greenhouse gas emissions, land and water use, and soil and air pollution [
38,
39]. However, AD plants are an underexploited source of proteins, which could be used in place of traditional protein sources, with a lower ecological footprint. AD plants, which at present are either run at a cost or in need of subsidies, could be turned into profitable or at least neutral-cost establishments, once equipped for the upcycling of proteins.
Proteins can be recovered from AD plants through different routes as described in
Figure 2:
The process of acidogenesis generates volatile fatty acids (VFA), which can easily be utilised as a source of carbon for the growth of different types of microorganisms. These are unicellular bacteria, fungi, yeasts and algae, which are rich in proteins and appear in the market under the name of microbial proteins (MP) or single cell proteins (SCP) once they have been dried and processed [
40]. At present, established technologies are already producing microbial proteins at competitive conversion rates, which are marketed with the required certifications and patents [
41].
CH
4, CO
2 and H
2 produced by methanogenesis are generally turned into thermal or electrical energy. However, these gases can be upcycled into microbial protein production by methanotrophic and hydrogenotrophic microbial strains, while the digestate can also be utilised as a source of nitrogen in the form of ammonia [
42,
43]. In the AD context, the CO
2 used as feedstock for bacterial strains can be generated from the upgrading of biogas into biomethane, or from the CO
2 emitted as a bioproduct of the combustion of biogas.
A more straightforward route for the recycling of proteins in AD plants is the utilisation of the microbial biomass produced in the digesters directly as MP source. This biomass containing mixed microbial cultures can be richer in protein than pure cultures, and has many applications, mainly in the animal feed sector [
44].
In this study, route 1 is followed: specifically, VFA generated in the acidogenic anaerobic fermenter is used to produce PHA-accumulating microbial biomass which can be employed for feeding purposes, especially in the aquaculture sector. Expected yields are high and interesting with microbial biomass production in the range of 0.3–0.5 kg VSS per kg COD consumed.
3.6. Expected Mass Balance for the Overall Biorefinery Process
The overall biorefinery process allows for the recovery of biofuels (hydrogen and methane, VFA, PHA and nutrients). PHA-rich microbial mass can be used as protein microbial material instead of as a source of polyesters.
Considering the feeding of 1 m
3 per day at 20% dry matter, the expected productions of hydrogen, methane, VFAs, PHAs, nitrogen and phosphorus are those reported in
Table 2.
The overall feedstock is anaerobically fermented and can produce some 40 kg of short chain fatty acids, where relative concentrations of acetic, propionic, butyric and valeric acids can be tuned using different operational conditions in terms of HRT and OLR. During the anaerobic process, up to 2 m3 of hydrogen is produced.
Produced VFAs can be considered a product itself, or, after solid/liquid separation and further purification, can be fed to the PHA-accumulating bioreactors. If transferred to this section, VFAs can produce up to 20 kg of microbial biomass containing up to 8 kg of PHAs. Because of the recovery procedure, the PHAs effectively recovered will be clearly lower than the calculated mass.
The solid fraction recovered after the solid/liquid separation step, equivalent to 60% of the total solids in the feedstock, is sent to the anaerobic digestion unit where up to 18 m3 methane can be produced. Anaerobic digestate will be then treated to separate solids and recover nutrients from the liquid phase via screening, microfiltration and ultrafiltration: 0.6 kg and 0.05 kg of nitrogen and phosphorus, respectively, can be recovered.
Table 2 summarizes the expected quantities of products.
4. Conclusions
Currently, more than 18,000 anaerobic digestion units are under operation in EU, 80% of which are employed in the agricultural sector. Because of the need for the sustainable production of biobased products from agro-waste and since the tariff schemes are rapidly approaching their end in several countries, there is the urgent need for a reconfiguration of these plants. In particular, these can be transformed into biorefineries able to transform agro-waste into valuable bio-based products.
In this study, a complete biorefinery platform for the treatment of agro-waste (especially cow manure) was designed and implemented at pilot scale in a real farm environment: biofuels (methane and hydrogen), VFAs, PHAs or microbial mass proteins were the main products. The high flexibility of the process allows for the different production of biofuels or biochemicals depending on the operator choices.
Considering the treatment of 1 m3 of slurry at 20% dry matter every day, for biofuels, up to 2 and 18 m3 of hydrogen and methane, respectively, can be produced. Then, 40 kg of VFA can be produced and up to 8 kg PHA after extraction and purification. Moreover, 0.6 kg and 0.05 kg of N and P, respectively, can be recovered in a concentrated form, easy to transported.
The practical set up of this platform will be further studied and its applicability will be analyzed especially through the environmental, social and economic benefits and sustainability performance that will be quantitatively assessed during the AgriLoop project implementation.