The need for environmentally friendly fuels for energy has recently been steadily increasing. Gas-to-liquid (GTL) is a technological breakthrough that uses the Fischer–Tropsch (FT) process to transform natural gas (NG) into high-performance, ultra-clean liquid fuels. This innovative and rising technology is likely to contribute to a higher proportion of global gas processing in the future. The development of GTL technology has advanced technologically over the past ten years, and many commercial-scale plants have been constructed across the world [
1]. GTL process water (PW) is a by-product of the Fischer–Tropsch (FT) reaction that produces water in significant quantities (~25%), more than other hydrocarbon products on a weight basis. Process water produced by GTL industries must be treated to meet the regulatory agency standards for both safe releases into water bodies and effective reuse [
2]. The majority of COD in the GTL PW water stream comes from alcohols, aldehydes, and ketones, which can be effectively treated biologically.
Biological approaches are popular in the wastewater treatment field because of their ease of use, environmental friendliness, low cost, and long-term application [
3]. Biological approaches involve using organisms such as bacteria, fungi, and algae to mineralize or break down contaminants into less toxic compounds [
4]. The use of bacteria is beneficial since these microorganisms are flexible representatives and contain a variety of Actinomycetes [
5]. Furthermore, these bacteria can produce spores, resist different contaminants, and survive in several environments. The organic pollutants in the GTL PW can be effectively treated biologically under aerobic and anaerobic conditions. The course of treatment may also combine anaerobic and aerobic systems. Several studies on GTL PW, namely FT wastewater, have been investigated on the bench and laboratory scales using both synthetic and actual wastewater, and biological treatment has been deliberated under anaerobic conditions [
6]. GTL PW is usually treated using a well-known biological process in conventional activated sludge (CAS) systems. This method of treatment is based on biomass, which is held by a settler, aerobically degrading organic contaminants. Three steps of GTL PW treatment, including chemical, biological, and physical treatment approaches, were described by Pon Saravanan and Van Vuuren [
2]. The three integrated processes of the GTL PW treatment plant used chemical treatment in the first stage to remove free oil and suspended hydrocarbons, followed by biotreatment in an aeration tank to eliminate carbonic and nitrogenous compounds, and finally physical treatment such as sand filtration in the third stage to eliminate suspended solids, oil, chemical oxygen demand, and related biological oxygen demand [
2]. The CAS process was combined with ultrafiltration (CAS-UF) and used in the treatment of GTL PW. The CAS effluent can undergo post-treatment ultrafiltration (UF) to remove unsettled particles and further lower the COD in the effluent, allowing for the reuse of the treated water [
7]. Additionally, Fischer–Tropsch (FT) reaction water from gas-to-liquid (GTL) industries was also treated using a membrane bioreactor (MBR) system and compared to the current treatment system [
7]. Majone et al. [
8] investigated the anaerobic biodegradation of synthetic FT wastewater with a high concentration of COD (~28 g/L) generated by long-chain alcohols utilizing a continuous flow-packed bed biofilm reactor (FPBBR) on a laboratory scale. They steadily increased the COD content in tests to evaluate the inhibitory effect of long-chain alcohol concentrations and achieved about 96% of COD reduction. Aerobic degradation of FT wastewater was studied by Chain et al. [
9] to decrease the high COD specifically from short-chain alcohols and volatile fatty acids as they represented around 87% of the given wastewater. The FT wastewater was synthesized from SCAs and VFAs in a mineral salt solution and a COD of 67.9 g/L and biodegraded using
Bacillus sp. Within 3 days, the strains reduced COD by up to 90% and effectively degraded the organic pollutants. Other studies [
6,
10] have suggested a combination of anaerobic biological treatment and chemical strategies to address the issue of incomplete degradation for long-chain alcohol under high organic load. Bio-electrochemical systems (BES) have been used in the treatment of FT wastewater by utilizing electrochemically active bacteria as a catalyst for oxidation and/or reduction processes at the anode and/or the cathode [
6]. By combining an anaerobic digester with a BES, the treatment performance was improved as well as the production of biogas [
11].
In most biological treatment processes of GTL PW, activated sludge is used as a biomass source where the biological community, considered as a mixed culture, contains a wide range of bacteria, fungi, and algae. Despite the biodegradability of these microorganisms, several types of pure cultures have been used over the years for the biodegradation of organic contaminants.
Pseudomonas was first identified as a Gram-negative, polar-flagellated, and rod-shaped bacteria by Migula in 1894, during the 19th century [
12]. It is one of the prokaryote genera that has undergone the most research (bacteria).
Pseudomonas aeruginosa is a Gram-negative bacterium that can be found in almost any environment. Their metabolic capacity is vast, as evidenced by their ability to synthesize a wide range of secondary metabolites and polymers as well as their ability to employ a wide range of carbon sources and electron acceptors [
13]. Many researchers have studied the ability of
P. aeruginosa to degrade various chemicals in the batch and continuous bioreactors under various conditions. These include the degradation of 0.02% naphthalene [
14], the biodegradation of volatile organic compounds [
15], the biodegradation of petroleum compounds [
16], the degradation of octamethylcyclotetrasiloxane (D4) [
17], and the bioremediation of heavy metals [
18]. However, the application of
P. aeruginosa for the biotreatment of GTL PW has never been previously documented. Hence, this study aimed to investigate the biodegradation of GTL water by
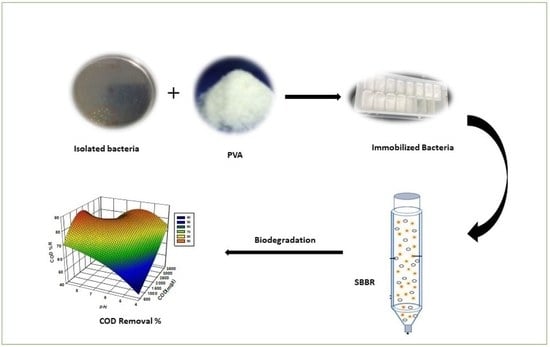
P. aeruginosa bacteria using a laboratory-scale spouted bed bioreactor system (SBBS). Moreover, the interaction between the operating parameters and the optimal conditions for the maximum reduction efficiencies by varying test parameters such as the initial COD concentration, PVA v%, and pH using RSM was investigated.