A Europium-Based Optical Sensor for the Detection of Carbon Dioxide and Its Application for a Fermentation Reaction
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Physical Measurements
2.3. Synthesis of K[Eu(hfa)4]
2.4. CO2 Purging Studies
2.5. RTIL (Room Temperature Ionic Liquid) Studies
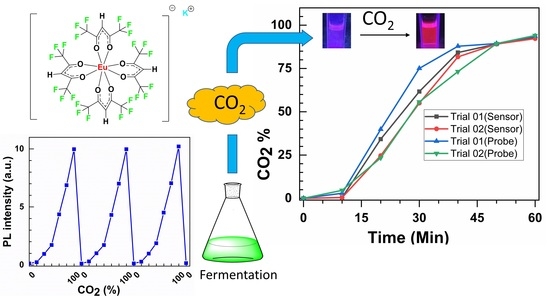
2.6. Fermentation
3. Results and Discussion
3.1. Crystal Structure and Complex Formula
3.2. “On–Off” pH-Switched Sensitization of K[Eu(hfa)4] PL
3.3. PL Titration and Determination of Acidity Constant (pKa)
3.4. CO2 Sensing Properties in Solution
3.5. Standardization and Quantification of the Sensor
3.6. A Blind Study to Determine Unknown Concentrations of CO2 Using the Standard Curve Equation
3.7. Interferences
3.8. Understanding the Changes in Molecular Dynamics after CO2 Purge
3.9. Evaluating K[Eu(hfa)4] for a Real-World Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Zhang, X.; Zhang, X.; Mu, H.; Li, H.; An, M.; Yoon, J.; Yu, H. Dual-channel sensing of CO2: Reversible solution-gel transition and gelation-induced fluorescence enhancement. Sens. Actuators B Chem. 2018, 255, 2764–2778. [Google Scholar] [CrossRef]
- Borchert, N.B.; Kerry, J.P.; Papkovsky, D.B. A CO2 sensor based on Pt-porphyrin dye and FRET scheme for food packaging applications. Sens. Actuators B Chem. 2013, 176, 157–165. [Google Scholar] [CrossRef]
- Lochman, L.; Zimcik, P.; Klimant, I.; Novakova, V.; Borisov, S.M. Red-emitting CO2 sensors with tunable dynamic range based on pH-sensitive azaphthalocyanine indicators. Sens. Actuators B Chem. 2017, 246, 1100–1107. [Google Scholar] [CrossRef]
- Sun, J.; Ye, B.; Xia, G.; Zhao, X.; Wang, H. A colorimetric and fluorescent chemosensor for the highly sensitive detection of CO2 gas: Experiment and DFT calculation. Sens. Actuators B Chem. 2016, 233, 76–82. [Google Scholar] [CrossRef]
- Contreras-Gutierrez, P.K.; Medina-Rodríguez, S.; Medina-Castillo, A.L.; Fernandez-Sanchez, J.F.; Fernandez-Gutierrez, A. A new highly sensitive and versatile optical sensing film for controlling CO2 in gaseous and aqueous media. Sens. Actuators B Chem. 2013, 184, 281–287. [Google Scholar] [CrossRef]
- Mills, A.; Lepre, A.; Wild, L. Breath-by-breath measurement of carbon dioxide using a plastic film optical sensor. Sens. Actuators B Chem. 1997, 39, 419–425. [Google Scholar] [CrossRef]
- Habib, M.; Hussain, S.S.; Riaz, S.; Naseem, S. Preparation and Characterization of ZnO Nanowires and their Applications in CO2 Gas Sensors. Mater. Today Proc. 2015, 2, 5714–5719. [Google Scholar] [CrossRef]
- Fernández-Sánchez, J.F.; Cannas, R.; Spichiger, S.; Steiger, R.; Spichiger-Keller, U.E. Optical CO2-sensing layers for clinical application based on pH-sensitive indicators incorporated into nanoscopic metal-oxide supports. Sens. Actuators. B Chem. 2007, 128, 145–153. [Google Scholar] [CrossRef]
- Ge, X.; Kostov, Y.; Rao, G. High-stability non-invasive autoclavable naked optical CO2 sensor. Biosens. Bioelectron. 2003, 18, 857. [Google Scholar] [CrossRef]
- Mills, A.; Chang, Q. Fluorescence plastic thin-film sensor for carbon dioxide. Analyst 1993, 118, 839–843. [Google Scholar] [CrossRef]
- Nakamura, N.; Amao, Y. An optical sensor for CO2 using thymol blue and europium(III) complex composite film. Sens. Actuators B Chem. 2003, 92, 98–101. [Google Scholar] [CrossRef]
- Chu, C.; Lo, Y. Fiber-optic carbon dioxide sensor based on fluorinated xerogels doped with HPTS. Sens. Actuators. B Chem. 2008, 129, 120–125. [Google Scholar] [CrossRef]
- Sipior, J.; Randers-Eichhorn, L.; Lakowicz, J.R.; Carter, G.M.; Rao, G. Phase Fluorometric Optical Carbon Dioxide Gas Sensor for Fermentation Off-Gas Monitoring. Biotechnol. Prog. 1996, 12, 266–271. [Google Scholar] [CrossRef]
- Lakowlcz, J.R.; Szmacmslu, H.; Karakelle, M. Optical sensing of pH and pC0, using phase-modulation fluorimetry and resonance energy transfer. Anaiytrca Chmrea Acta 1993, 272, 179. [Google Scholar] [CrossRef]
- Agayn, V.I.; Walt, D.R. Fiber-optic Sensor for Continuous Monitoring of Fermentation pH. Bio/Technol. 1993, 11, 726–729. [Google Scholar] [CrossRef]
- Leiner, M.J.P. Luminescence chemical sensors for biomedical applications: Scope and limitations. Anal. Chim. Acta 1991, 255, 209. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Sato, N.; Hirai, Y.; Nakanishi, T.; Kitagawa, Y.; Kobayashi, A.; Kato, M.; Seki, T.; Ito, H.; Fushimi, K. Enhanced Electric Dipole Transition in Lanthanide Complex with Organometallic Ruthenocene Units. J. Phys. Chem. A 2015, 119, 4825–4833. [Google Scholar] [CrossRef]
- Lunstroot, K.; Driesen, K.; Nockemann, P.; Van Hecke, K.; Van Meervelt, L.; Görller-Walrand, C.; Binnemans, K.; Bellayer, S.; Viau, L.; Le Bideau, J.; et al. Ionic liquid as plasticizer for europium(III)-doped luminescent poly(methyl methacrylate) films. Phys. Chem. Chem. Phys. 2010, 12, 1879–1885. [Google Scholar] [CrossRef] [Green Version]
- Parker, D. Luminescent lanthanide sensors for pH, pO 2 and selected anions. Coord. Chem. Rev. 2000, 205, 109–130. [Google Scholar] [CrossRef] [Green Version]
- Lowe, M.; Parker, D.; Reany, O.; Aime, S.; Botta, M.; Castellano, G.; Gianolio, E.; Pagliarin, R. pH-dependent modulation of relaxivity and luminescence in macrocyclic gadolinium and europium complexes based on reversible intramolecular sulfonamide ligation. J. Am. Chem. Soc. 2001, 123, 7601–7609. [Google Scholar] [CrossRef]
- Zhang, X.; Jiao, Y.; Jing, X.; Wu, H.; He, G.; Duan, C. pH-sensitive fluorescent sensors based on europium(III) complexes. Dalton Trans. 2011, 40, 2522. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wei, H.; Yan, W.; Zhao, Z.; Cai, Z.; Sun, B.; Meng, Z.; Liu, Z.; Bian, Z.; Huang, C. Water-Soluble and Highly Luminescent Europium(III) Complexes with Favorable Photostability and Sensitive pH Response Behavior. Inorg. Chem. 2016, 55, 10645–10653. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, R.A.; Delgado-Alonso, J. Extended-length fiber optic carbon dioxide monitoring. SPIE 2013, 8718, 79–88. [Google Scholar]
- Ge, X.; Kostov, Y.; Rao, G. Low-cost noninvasive optical CO2 sensing system for fermentation and cell culture. Biotechnol. Bioeng. 2005, 89, 329–334. [Google Scholar] [CrossRef]
- Marques, W.L.; Raghavendran, V.; Stambuk, B.U.; Gombert, A.K. Sucrose and Saccharomyces cerevisiae: A relationship most sweet. FEMS Yeast Res. 2016, 16, fov107. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.; Miller, A.; Hoang, A.; Collette, J.; Dubose, T. Sucrose increases the emission of CO2 during yeast (Saccharomyces cerevisiae) fermentation faster than glucose. J. Undergrad. Biol. Lab. Investig. 2019, 2, 1–4. [Google Scholar]
- Crancer, M.; Roy, J.; Zayaleta, C.; Hjalmerson, E. Analyzing the Rate of Carbon Dioxide Created by Fermentation in Yeast With Different Types of Sugars. J. Undergrad. Biol. Lab. Investig. 2018, 1, 1–5. [Google Scholar]
- De Silva, C.R.; Wang, R.; Zheng, Z. Highly luminescent Eu(III) complexes with 2,4,6-tri(2-pyridyl)-1,3,5-triazine ligand: Synthesis, structural characterization, and photoluminescence studies. Polyhedron 2006, 25, 3449–3455. [Google Scholar] [CrossRef]
- Koutsokali, M.; Valahas, M. Anaerobic and Aerobic Respiration in Yeast: Small-Scale Variations on a Classic Laboratory Activity. J. Chem. Educ. 2020, 97, 1041–1047. [Google Scholar] [CrossRef]
- Burns, J.H.; Danford, M.D. The Crystal Structure of Cesium Tetrakis (hexafluoroacetylacetonato)europate and -americate. Isomorphism with the Yttrate. Inorg. Chem. 1968, 8, 1780–1784. [Google Scholar] [CrossRef]
- Barry, M.C.; Wei, Z.; He, T.; Filatov, A.S.; Dikarev, E.V. Volatile Single-Source Precursors for the Low-Temperature Preparation of Sodium-Rare Earth Metal Fluorides. J. Am. Chem. Soc. 2016, 138, 8883–8887. [Google Scholar] [CrossRef]
- Armelao, L.; Belli Dell’Amico, D.; Bottaro, G.; Bellucci, L.; Labella, L.; Marchetti, F.; Mattei, C.A.; Mian, F.; Pineider, F.; Poneti, G.; et al. 1D hetero-bimetallic regularly alternated 4f-3d coordination polymers based on N-oxide-4,4′-bipyridine (bipyMO) as a linker: Photoluminescence and magnetic properties. Dalton Trans. 2018, 47, 8337–8345. [Google Scholar] [CrossRef] [Green Version]
- Osawa, M.; Hoshino, M.; Wada, T.; Hayashi, F.; Osanai, S. Intra-Complex Energy Transfer of Europium(III) Complexes Containing Anthracene and Phenanthrene Moieties. J. Phys. Chem. A 2009, 113, 10895–10902. [Google Scholar] [CrossRef]
- Kozuka, T.; Yamamoto, M.; Ktagawa, Y.; Nakansh, T.; Fushm, K.; Hasegawa, Y. Photosensitized Luminescence of Highly Thermostable Mononuclear Eu(III) Complexes with π-Expanded β-Diketonate Ligands. Bull. Chem. Soc. Jpn. 2017, 90, 1287–1292. [Google Scholar] [CrossRef]
- Yamamoto, M.; Nakansh, T.; Ktagawa, Y.; Sek, T.; Ito, H.; Fushm, K.; Hasegawa, Y. Synthesis and Photophysical Properties of Eu(III) Complexes with Phosphine Oxide Ligands including Metal Ions. Bull. Chem. Soc. Jpn. 2018, 91, 6–11. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, N.; Li, H.; Chen, P.; Yan, P. The Role of Blue-Emissive 1,8-Naphthalimidopyridine N-Oxide in Sensitizing EuIII Photoluminescence in Dimeric Hexafluoroacetylacetonate Complexes. Eur. J. Inorg. Chem. 2017, 2017, 2211–2219. [Google Scholar] [CrossRef]
- Lyle, S.J.; Witts, A.D. A Critical Examination of Some Methods for the Preparation of Tris and Tetrakis Diketonates of Europium(III). Inorg. Chem. Acta 1971, 5, 481–484. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Yamamuro, M.; Wada, Y.; Kanehisa, N.; Kai, Y.; Yanagida, S. Luminescent Polymer Containing the Eu(III) Complex Having Fast Radiation Rate and High Emission Quantum Efficiency. J. Phys. Chem. A 2003, 107, 1697–1702. [Google Scholar] [CrossRef]
- Richardson, M.F.; Wagner, W.F.; Sands, D.E. Rare-earth trishexafluoroacetylacetonates and related compounds. J. Inorg. Nucl. Chem. 1968, 30, 1275–1289. [Google Scholar] [CrossRef]
- Cotton, S. Coordination Chemistry of the Lanthanides. In Lanthanide and Actinide Chemistry; Woolins, D., Crabtree, B., Atwood, D., Meyer, G., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2006; pp. 39–41. [Google Scholar]
- Langer, V.; Smrčok, Ľ.; Boča, M. Redetermination of Na3TaF8. Acta Crystallogr. C 2010, 66, i8–i86. [Google Scholar] [CrossRef]
- Peterson, S.W.; Holloway, J.H.; Coyle, B.A.; Williams, J.M. Antiprismatic Coordination about Xenon: The Stucture of Nitrosonium Octafluoroxenate(VI). Science 1971, 173, 1238–1239. [Google Scholar] [CrossRef] [PubMed]
- Gunnlaugsson, T.; Parker, D. Luminescent europium tetraazamacrocyclic complexes with wide range pH sensitivity. Chem. Commun. 1998, 4, 511–512. [Google Scholar] [CrossRef]
- Lowe, M.P.; Parker, D. pH Switched sensitisation of europium(III) by a dansyl group. Inorg. Chim. Acta 2001, 317, 163–173. [Google Scholar] [CrossRef]
- Bazzicalupi, C.; Bencini, A.; Bianchi, A.; Giorgi, C.; Masotti, A.; Valtancoli, B.; Fusi, V.; Roque, A.; Pina, F. pH Modulation of The Luminescence Emission of a New Europium Cryptate Complex. Chem. Commun. 2000, 7, 561–562. [Google Scholar] [CrossRef]
- Gunnlaugsson, T.; Leonard, J.P.; Sénéchal, K.; Harte, A.J. pH Responsive Eu(III)−Phenanthroline Supramolecular Conjugate: Novel “Off − On − Off” Luminescent Signaling in the Physiological pH Range. J. Am. Chem. Soc. 2003, 125, 12062–12063. [Google Scholar] [CrossRef]
- Sabbatini, N.; Ciano, M.S.; Dellonte, S.; Bonazzi, A.; Balzani, V. Absorption and emission properties of a europium(II) cryptate in aqueous solution. Chem. Phys. Lett. 1982, 90, 265. [Google Scholar] [CrossRef]
- Sabbatini, N.; Ciano, M.; Dellonte, S.; Bonazzi, A.; Bolletta, F.; Balzani, V. Photophysical properties of europium(II) cryptates. J. Phys. Chem. 1984, 88, 1534–1537. [Google Scholar] [CrossRef]
- Ishii, A.; Hasegawa, M. An Interfacial Europium Complex on SiO2 Nanoparticles: Reduction-Induced Blue Emission System. Sci. Rep. 2015, 5, 11714. [Google Scholar] [CrossRef]
- Christoffers, J.; Starynowicz, P. A europium(II) complex with bis-pyridino-18-crown-6. Polyhedron 2008, 27, 2688–2692. [Google Scholar] [CrossRef]
- Sadi, B.B.; Li, C.; Kramer, G.H. An emergency radiobioassay method for 226Ra in human urine samples. Radiat. Prot. Dosim. 2012, 151, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Jin, J.; Zhang, L.; Li, W. Absorption of carbon dioxide into aqueous blend of monoethanolamine and 1-butyl-3-methylimidazolium tetrafluoroborate. Int. J. Greenh. Gas Control 2012, 11, 152–157. [Google Scholar] [CrossRef]
- Kobos, R.K.; Parks, S.J.; Meyerhoff, M.E. Selectivity Characteristics of Potentiometric Carbon Dioxide Sensors with Various Gas Membrane Materials. Anal. Chem. 1982, 54, 1976–1980. [Google Scholar] [CrossRef]
- Mascini, M.; Cremisini, C. Evaluation of measuring range and interferences for gas-sensing potentiometric probes. Anal. Chim. Acta 1978, 97, 237. [Google Scholar] [CrossRef]
- Revsbech, N.P.; Garcia-Robledo, E.; Sveegaard, S.; Andersen, M.H.; Gothelf, K.V.; Larsen, L.H. Amperometic microsensor for measurement of gaseous and dissolved CO2. Sens. Actuators. B Chem. 2019, 283, 349–354. [Google Scholar] [CrossRef]
- Ishiji, T.; Takahashi, K.; Kira, A. Amperometric carbon dioxide gas sensor based on electrode reduction of platinum oxide. Anal. Chem. 1993, 65, 2736–2739. [Google Scholar] [CrossRef]
- Malandrino, G.; Bettinelli, M.; Speghini, A.; Fragalà, I.L. Europium ”Second Generation” Precursors for Metal-Organic Chemical Vapor Deposition: Characterization and Optical Spectroscopy. Eur. J. Inorg. Chem. 2001, 2001, 1039–1044. [Google Scholar] [CrossRef]
- Bray, R.G. Infrared and Raman Spectroscopy of Uranyl (Hexafluoroacetyl-Acetonate)2 Adducts in the Vapor and Condensed Phases. Spectrochim. Acta 1983, 39, 559. [Google Scholar] [CrossRef]
- Howard, D.L.; Kjaergaard, H.G.; Huang, J.; Meuwly, M. Infrared and Near-Infrared Spectroscopy of Acetylacetone and Hexafluoroacetylacetone. J. Phys. Chemistry. A Mol. Spectrosc. Kinet. Environ. Gen. Theory 1900, 119, 7980–7990. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2014, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]







| Gas Sample | %CO2, Calibration Curve | %CO2, Actual |
|---|---|---|
| A | 1.37% | 1.99% |
| B | 6.38% | 6.00% |
| C | 10.57% | 10.04% |
| Response Time, Min. | |
|---|---|
| N2 to 5% CO2 | 3 |
| 5% to 50% CO2 | 5 |
| 50% CO2 to 100% CO2 | 6 |
| 100% CO2 to N2 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benton, E.N.; Perera, N.A.K.R.; Nesterov, V.N.; Perera, W.; Omary, M.A.; Marpu, S.B. A Europium-Based Optical Sensor for the Detection of Carbon Dioxide and Its Application for a Fermentation Reaction. Chemosensors 2023, 11, 5. https://doi.org/10.3390/chemosensors11010005
Benton EN, Perera NAKR, Nesterov VN, Perera W, Omary MA, Marpu SB. A Europium-Based Optical Sensor for the Detection of Carbon Dioxide and Its Application for a Fermentation Reaction. Chemosensors. 2023; 11(1):5. https://doi.org/10.3390/chemosensors11010005
Chicago/Turabian StyleBenton, Erin N., Nawagamu A. K. Rajitha Perera, Vladimir N. Nesterov, Wijayantha Perera, Mohammad A. Omary, and Sreekar B. Marpu. 2023. "A Europium-Based Optical Sensor for the Detection of Carbon Dioxide and Its Application for a Fermentation Reaction" Chemosensors 11, no. 1: 5. https://doi.org/10.3390/chemosensors11010005