Nanocomposites of Carbon Quantum Dots and Graphene Quantum Dots: Environmental Applications as Sensors
Abstract
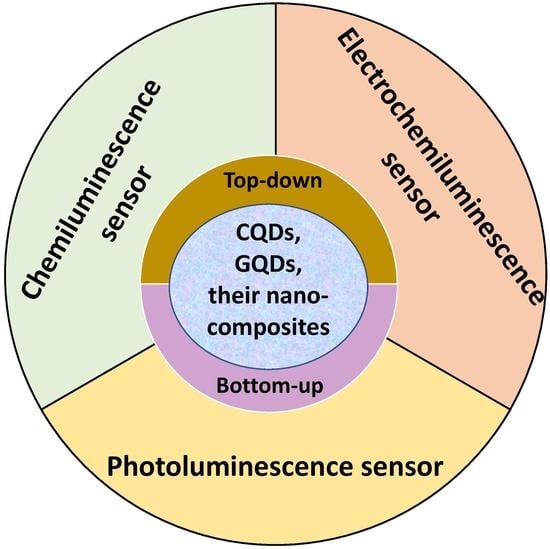
:1. Introduction
2. CQDs and GQDs
3. Difference between CQDs and GQDs
4. Synthesis of CQDs, GQDs and Their Nanocomposites
4.1. Top-Down Methods for the Synthesis of CQDs and Their Nanocomposites
4.2. Bottom-Up Methods for Thesynthesis of CQDs and Their Nanocomposites
4.3. Top-Down Methods for Synthesis of GQDs and Their Nanocomposites
4.4. Bottom-Up Method for Synthesis of GQDs and Their Nanocomposites
4.5. Synthesis of CQDs, GQDs, and Their Nanocomposites from Natural Products
5. Sensing Applications
5.1. CQDs and Their Nanocomposites as Sensing Platforms
5.2. GQDs and Their Nanocomposites as Sensing Platforms
5.3. Mechanism of Sensing
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, G.; Kumar, A.; Naushad, M.; Kumar, A.; Al-Muhtaseb, A.H.; Dhiman, P.; Ghfar, A.A.; Stadler, F.J.; Khan, M.R. Photoremediation of toxic dye from aqueous environment using monometallic and bimetallic quantum dots-based nanocomposites. J. Clean. Prod. 2018, 172, 2919–2930. [Google Scholar] [CrossRef]
- Singh, P.; Shandilya, P.; Raizada, P.; Sudhaik, A.; Rahmani-Sani, A.; Hosseini- Bandegharaei, A. Review on various strategies for enhancing photocatalytic activity of graphene-based nanocomposites for water purification. Arab. J. Chem. 2020, 13, 3498–3520. [Google Scholar] [CrossRef]
- Reshma, V.G.; Mohanan, P.V. Quantum dots: Applications and safety consequences. J. Lumin. 2019, 205, 287–298. [Google Scholar] [CrossRef]
- Hu, Z.M.; Fei, G.T.; Zhang, L.D. Synthesis and tunable emission of Ga2S3 quantumdots. Mater. Lett. 2019, 239, 17–20. [Google Scholar] [CrossRef]
- Hagiwara, K.; Horikoshi, S.; Serpone, N. Luminescent monodispersed carbon quantum dots by a microwave solvothermal method toward bioimaging applications. J. Photochem. Photobiol. A Chem. 2021, 415, 113310. [Google Scholar] [CrossRef]
- Ding, S.; Gao, Y.; Ni, B.; Yang, X. Green synthesis of biomass-derived carbon quantum dots as fluorescent probe for Fe3+ detection. Inorg. Chem. Commun. 2021, 130, 108636. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Sedighi, O.; Tabatabaei Rezaei, N.; Abbas Abedini, A.; Malek Khachatourian, A.; Toprak, M.S.; Seifalian, A. Recent advances in the modification of carbon-based quantum dots for biomedical applications. Mater. Sci. Eng. C 2021, 120, 111756. [Google Scholar] [CrossRef]
- Yue, X.-Y.; Zhou, Z.-J.; Wu, Y.-M.; Li, Y.; Li, J.-C.; Bai, Y.-H.; Wang, J.-L. Application Progress of Fluorescent Carbon Quantum Dots in Food Analysis. Chin. J. Anal. Chem. 2020, 48, 1288–1296. [Google Scholar] [CrossRef]
- Abd Rani, U.; Yong Ng, L.; Yin Ng, C.; Mahmoudi, E. A review of carbon quantum dots and their applications in wastewater treatment. Adv. Colloid Interface Sci. 2020, 278, 102124. [Google Scholar] [CrossRef]
- Rahal, M.; Atassi, Y.; Alghoraibi, I. Quenching photoluminescence of Carbon Quantum Dots for detecting and tracking the release of Minocycline. J. Photochem. Photobiol. A Chem. 2021, 412, 113257. [Google Scholar] [CrossRef]
- Wei Heng, Z.; Chan Chong, W.; Ling Pang, Y.; Hoon Koo, C. An overview of the recent advances of carbon quantum dots/metal oxides in the application of heterogeneous photocatalysis in photodegradation of pollutants towards visible-light and solar energy exploitation. J. Environ. Chem. Eng. 2021, 9, 105199. [Google Scholar] [CrossRef]
- Ju-Meng, W.; Bi-Tao, L.; Xin, Z.; Chang-Chun, S. One-pot synthesis of N, S co-doped photoluminescent carbon quantum dots for Hg2+ ion detection. New Carbon Mater. 2018, 33, 333–340. [Google Scholar]
- Cirone, J.; Rahin Ahmed, S.; Wood, P.C.; Chen, A. Green synthesis and electrochemical study of cobalt/graphene quantum dots for efficient water splitting. J. Phys. Chem. C 2019, 123, 9183–9191. [Google Scholar] [CrossRef]
- Shin, J.; Guo, J.; Zhao, T.; Guo, Z. Functionalized carbon dots on graphene as outstanding non-metal bifunctional oxygen electrocatalyst. Small 2019, 15, 1900296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Bin, D.; Wei, J.-S.; Niu, X.-Q.; Chen, X.-B.; Xia, Y.-Y.; Xiong, H.-M. Efficient oxygen electrocatalyst for Zn-air batteries: Carbon dots and Co9S8nanoparticles in a N, S-codoped carbon matrix. ACS Appl. Mater. Interfaces 2019, 11, 14085–14094. [Google Scholar] [CrossRef]
- Ding, H.; Zhou, X.-X.; Zhang, Z.-H.; Zhao, Y.-P.; Wei, J.-S.; Xiong, H.-M. Large scale synthesis of full-color emissive carbon dots from a single carbon source by a solvent-free method. Nano Res. 2021, 15, 3548–3555. [Google Scholar] [CrossRef]
- Liu, L.; Miao, Q.; Liang, G. Quantum dots as multifunctional materials for tumor imaging and therapy. Materials 2013, 6, 483–499. [Google Scholar] [CrossRef]
- Chen, W.; Li, D.; Tian, L.; Xiang, W.; Wang, T.; Hu, W.; Hu, Y.; Chen, S.; Chen, J.; Dai, Z. Synthesis of graphene quantum dots from natural polymer starch for cell imaging. Green Chem. 2018, 20, 4438–4442. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, F.; Hu, S.; Wu, B.; Wang, Y.; Zhang, H.; Jiang, B.; Fu, H. Graphene quantum-dot-modified hexagonal tubular carbon nitride for visible-light photocatalytic hydrogen evolution. ChemCatChem 2018, 10, 1330–1335. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Ahmad Nazri, N.A.; HidayahAzeman, N.; Luo, Y.; Ashrif, A.; Bakar, A. Carbon quantum dots for optical sensor applications: A review. Opt. Laser Technol. 2021, 139, 106928. [Google Scholar] [CrossRef]
- Kumar Barman, M.; Patra, A. Current status and prospects on chemical structure driven photoluminescence behaviour of carbon dots. J. Photochem. Photobiol. C 2018, 37, 1–22. [Google Scholar] [CrossRef]
- Ying Lim, S.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. 2015, 44, 362–381. [Google Scholar]
- Yu, X.; Liu, J.; Yu, Y.; Zuo, S.; Li, B. Preparation and visible light photocatalytic activity of carbon quantum dots/TiO2 nanosheet composites. Carbon 2014, 68, 718–724. [Google Scholar] [CrossRef]
- JafarMolaei, M. Principles, mechanisms, and application of carbon quantum dots in sensors: A review. Anal. Methods 2020, 12, 1266–1287. [Google Scholar]
- Zhu, J.; Tang, Y.; Wang, G.; Mao, J.; Liu, Z.; Sun, T.; Wang, M.; Chen, D.; Yang, Y.; Li, J.; et al. Green, rapid, and universal preparation approach of graphene quantum dots under ultraviolet irradiation. ACS Appl. Mater. Interfaces 2017, 9, 14470–14477. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, L.; Ruan, Y.-F.; Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Quantum-dots-based photoelectrochemical bioanalysis highlighted with recent examples. Biosens. Bioelectron. 2017, 94, 207–218. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, X.; Song, F.; Wang, C.; Chu, F.; Wu, S. A sensing approach for dopamine determination by boronic acid- functionalized molecularly imprinted graphene quantum dots composite. Appl. Surf. Sci. 2017, 423, 810–816. [Google Scholar] [CrossRef]
- Gong, P.; Wang, J.; Hou, K.; Yang, Z.; Wang, Z.; Liu, Z.; Han, X.; Yang, S. Small but strong: The influence of fluorine atoms on formation and performance of graphene quantum dots using a gradient F-sacrifice strategy. Carbon 2017, 112, 63–71. [Google Scholar] [CrossRef]
- Yuan, F.; Ding, L.; Li, Y.; Li, X.; Fan, L.; Zhou, S.; Fang, D.; Yang, S. Multicolor fluorescent graphene quantum dots colorimetrically responsive to all-pH and a wide temperature range. Nanoscale 2015, 7, 11727–11733. [Google Scholar] [CrossRef]
- Chandra Biswas, M.; Islam, T.; Kumar Nandy, P.; Hossain, M. Graphene Quantum Dots (GQDs) for bioimaging and drug delivery applications: A review. ACS Mater. Lett. 2021, 3, 889–911. [Google Scholar] [CrossRef]
- Fan, Q.; Li, J.; Zhu, Y.; Yang, Z.; Shen, T.; Guo, Y.; Wang, L.; Mei, T.; Wang, J.; Wang, X. Functional Carbon Quantum Dots towards Highly Sensitive Graphene Transistors for Cu2+ Ion Detection. ACS Appl. Mater. Interfaces 2020, 12, 4797–4803. [Google Scholar]
- Madurani, K.A.; Suprapto, S.; Syahputra, M.Y.; Puspita, I.; Masudi, A.; Rizqi, H.D.; Hatta, A.M.; Juniastuti, J.; Lusida, M.I.; Kurniawan, F. Recent development of detection methods for controlling COVID-19 outbreak. J. Electrochem. Soc. 2021, 168, 37511. [Google Scholar] [CrossRef]
- Li, Y.; Ma, P.; Tao, Q.; Krause, H.J.; Yang, S.; Ding, G.; Dong, H.; Xie, X. Magnetic graphene quantum dots facilitate closed-tube one-step detection of SARS-CoV-2 with ultra-low field NMR relaxometry. Sens. Actuators B Chem. 2021, 337, 129786. [Google Scholar] [CrossRef]
- Yuan, H.; Lin, J.-H.; Dong, Z.-S.; Chen, W.-T.; Chan, Y.K.; Yeh, Y.-C.; Chang, H.-T.; Chen, C.-F. Detection of pathogens using graphene quantum dots and gold nanoclusters on paper-based analytical devices. Sens. Actuators B Chem. 2022, 363, 131824. [Google Scholar] [CrossRef]
- Kuo, W.-S.; Chen, H.-H.; Chen, S.-Y.; Chang, C.-Y.; Chen, P.-C.; Hou, Y.-I.; Shao, Y.-T.; Kao, H.-F.; Lilian Hsu, C.-L.; Chen, Y.-C.; et al. Graphene quantum dots with nitrogen-doped content dependence for highly efficient dual-modality photodynamic antimicrobial therapy and bioimaging. Biomaterials 2017, 120, 185–194. [Google Scholar] [CrossRef]
- Feng, H.; Qian, Z. Functional carbon quantum dots: A versatile platform for chemosensing and biosensing. Chem. Rec. 2018, 18, 491–505. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. 2013, 125, 4045–4049. [Google Scholar] [CrossRef]
- Luo, Z.; Qi, G.; Chen, K.; Zou, M.; Yuwen, L.; Zhang, X.; Huang, W.; Wang, L. Microwave-Assisted Preparation of White Fluorescent Graphene Quantum Dots as a Novel Phosphor for Enhanced White-Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 2739–2744. [Google Scholar] [CrossRef]
- Martindale, B.C.M.; Hutton, G.A.; Caputo, C.A.; Reisner, E. Solar hydrogen production using carbon quantum dots and a molecular nickel catalyst. J. Am. Chem. Soc. 2015, 137, 6018–6025. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; MacFarlane, D.R. Carbon Quantum Dots/Cu2O Heterostructures for Solar-Light-Driven Conversion of CO2 to Methanol. Adv. Energy Mater. 2015, 5, 1401077. [Google Scholar] [CrossRef]
- Tuerhong, M.; Xu, Y.; Yin, X.-B. Review on Carbon Dots and Their Applications. Chin. J. Anal. Chem. 2017, 45, 139–150. [Google Scholar] [CrossRef]
- Fan, J.; Li, D.; Wang, X. Effect of modified graphene quantum dots on photocatalytic degradation property. Diam. Relat. Mater. 2016, 69, 81–85. [Google Scholar] [CrossRef]
- Li, H.; Liu, R.; Kong, W.; Liu, J.; Liu, Y.; Zhou, L.; Zhang, X.; Lee, S.-T.; Kang, Z. Carbon quantum dots with photo-generated proton property as efficient visible light controlled acid catalyst. Nanoscale 2013, 6, 867–873. [Google Scholar] [CrossRef]
- Li, M.; Wang, M.; Zhu, L.; Li, Y.; Yan, Z.; Shen, Z.; Cao, X. Facile microwave assisted synthesis of N-rich carbon quantum dots/dual-phase TiO2 heterostructured nanocomposites with high activity in CO2photoreduction. Appl. Catal. B 2018, 231, 269–276. [Google Scholar] [CrossRef]
- Ding, D.; Lan, W.; Yang, Z.; Zhao, X.; Chen, Y.; Wang, J.; Zhang, X.; Zhang, Y.; Su, Q.; Xie, E. A simple method for preparing ZnO foam/carbon quantum dots nanocomposite and their photocatalytic applications. Mater. Sci. Semicond. Process. 2016, 47, 25–31. [Google Scholar] [CrossRef]
- Zhang, H.; Ming, H.; Lian, S.; Huang, H.; Li, H.; Zhang, L.; Liu, Y.; Kang, Z.; Lee, S.-T. Fe2O3/carbon quantum dots complex photocatalysts and their enhanced photocatalytic activity under visible light. Dalton Trans. 2011, 40, 10822–10825. [Google Scholar] [CrossRef]
- Han, X.; Han, Y.; Huang, H.; Zhang, H.; Zhang, X.; Liu, R.; Liu, Y.; Kang, Z. Synthesis of carbon quantum dots/SiO2porous nanocomposites and their catalytic ability for photo- enhanced hydrocarbon selective oxidation. Dalton Trans. 2013, 42, 10380–10383. [Google Scholar] [CrossRef]
- Mosconi, D.; Mazzier, D.; Silvestrini, S.; Privitera, A.; Marega, C.; Franco, L.; Moretto, A. Synthesis and photochemical applications of processable polymers enclosing photoluminescent carbon quantum dots. ACS Nano 2015, 9, 4156–4164. [Google Scholar] [CrossRef]
- Huang, C.-C.; Hung, Y.-S.; Weng, Y.-M.; Chen, W.; Lai, Y.-S. Sustainable development of carbon nanodots technology: Natural products as a carbon source and applications to food safety. Trends Food Sci. Technol. 2019, 86, 144–152. [Google Scholar] [CrossRef]
- Bottini, M.; Tautz, L.; Huynh, H.; Monosov, E.; Bottini, N.; Dawson, M.I.; Bellucci, S.; Mustelin, T. Covalent decoration of multi-walled carbon nanotubes with silica nanoparticles. Chem. Commun. 2005, 6, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Sharma, N.N. Arc discharge synthesis of carbon nanotubes: Comprehensive review. Diam. Relat. Mater. 2014, 50, 135–150. [Google Scholar] [CrossRef]
- Biazar, N.; Poursalehi, R.; Delavari, H. Optical and Structural Properties of Carbon dots/TiO2 Nanostructures Prepared via DC arc Discharge in Liquid. In Proceedings of the 6th International Biennial Conference on Ultrafine Grained and Nanostructured Materials (UFGNSM), Kish Island, Iran, 12–13 November 2017; Sohi, M.H., Zamani, C., Eds.; AIP Publishing LLC: Melville, NY, USA, 2018. [Google Scholar]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; ShiralFernando, K.A.; Pathak, P.; JaouadMeziani, M.; Harruff, B.A.; Wang, X.; Wang, H.F.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Doñate-Buendia, C.; Torres-Mendieta, R.; Pyatenko, A.; Falomir, E.; Fernández-Alonso, M.; Mínguez-Vega, G. Fabrication by laser irradiation in a continuous flow jet of carbon quantum dots for fluorescence imaging. ACS Omega 2018, 3, 27352742. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, X.; Zeng, X.; Lu, Y. Preparation of carbon dots by non-focusing pulsed laser irradiation in toluene. Chem. Commun. 2016, 52, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-L.; Niu, K.-Y.; Sun, J.; Yang, J.; Zhao, N.-Q.; Du, X.-W. One-step synthesis of fluorescent carbon nanoparticles by laser irradiation. J. Mater. Chem. 2009, 19, 484–488. [Google Scholar] [CrossRef]
- Kazemizadeh, F.; Malekfar, R.; Parvin, P. Pulsed laser ablation synthesis of carbon nanoparticles in vacuum. J. Phys. Chem. Solids 2017, 104, 252–256. [Google Scholar] [CrossRef]
- Nguyen, V.; Zhao, N.; Yan, L.; Zhong, P.; CanhNguyen, V.; HuuLe, P. Double-pulse femtosecond laser ablation for synthesis of ultrasmall carbon nanodots. Mater. Res. Express 2020, 7, 015606. [Google Scholar] [CrossRef]
- Liu, H.; Ye, T.; Mao, C. Fluorescent carbon nanoparticles derived from candle soot. Angew. Chem. Int. Ed. 2007, 46, 6473–6475. [Google Scholar] [CrossRef]
- Peng, H.; Travas-Sejdic, J. Simple aqueous solution route to luminescent carbogenic dots from carbohydrates. Chem. Mater. 2009, 21, 5563–5565. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, X.; Ruan, H.; Yin, K.; Li, H. Production of yellow-emitting carbon quantum dots from fullerene carbon soot. Sci. China Mater. 2017, 60, 141–150. [Google Scholar] [CrossRef]
- Meng, X.; Chang, Q.; Xue, C.; Yang, J.; Hu, S. Full-colour carbon dots: From energy-efficient synthesis to concentration-dependent photoluminescence properties. Chem. Commun. 2017, 53, 3074–3077. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Liu, C.; Zhang, Z.-L.; Pang, D.-W. Photoluminescence-tunable carbon nanodots: Surface-state energy-gap tuning. Adv. Mater. 2015, 27, 1663–1667. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Han, X.-Y.; Lin, Z.-Y.; Fan, Y.-L.; Shi, G.; Zhang, S.; Zhang, M. Facile reflux synthesis of polyethyleneimine-capped fluorescent carbon dots for sequential bioassays toward Cu2+/H2S and its application for a logic system. Biotechnol. Appl. Biochem. 2019, 66, 426–433. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Liu, Y.; Huang, H.; Lian, S.; Lee, S.-T.; Kang, Z. One-step ultrasonic synthesis of water-soluble carbon nanoparticles with excellent photoluminescent properties. Carbon 2011, 49, 605–609. [Google Scholar] [CrossRef]
- Qiang, R.; Yang, S.; Hou, K.; Wang, J. Synthesis of carbon quantum dots with green luminescence from potato starch. New J. Chem. 2019, 43, 10826–10833. [Google Scholar] [CrossRef]
- Huang, H.; Cui, Y.; Liu, M.; Chen, J.; Wan, Q.; Wen, Y.; Deng, F.; Zhou, N.; Zhang, X.; Wei, Y. A one-step ultrasonic irradiation assisted strategy for the preparation of polymer-functionalized carbon quantum dots and their biological imaging. J. Colloid Interface Sci. 2018, 532, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhou, L. One-step sonochemical synthesis of versatile nitrogen-doped carbon quantum dots for sensitive detection of Fe2+ions and temperature in vitro. Mater. Sci. Eng. C-Mater. 2019, 101, 352–359. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, M.; Yong Kim, H.; Ding, B.; Park, S.-J. A facile ultrasonic-assisted fabrication of nitrogen-doped carbon dots/BiOBr up-conversion nanocomposites for visible light photocatalytic enhancements. Sci. Rep. 2017, 7, 45086. [Google Scholar] [CrossRef]
- Ma, Z.; Ming, H.; Huang, H.; Liu, Y.; Kang, Z. One-step ultrasonic synthesis of fluorescent N-doped carbon dots from glucose and their visible-light sensitive photocatalytic ability. New J. Chem. 2012, 36, 861–864. [Google Scholar] [CrossRef]
- Dang, H.; Huang, L.K.; Zhang, Y.; Wang, C.-F.; Chen, S. Large-scale ultrasonic fabrication of white fluorescent carbon dots. Ind. Eng. Chem. Res. 2016, 55, 5335–5341. [Google Scholar]
- Suzuki, K.; Malfatti, L.; Carboni, D.; Loche, D.; Casula, M.; Moretto, A.; Maggini, M.; Takahashi, M.; Innocenzi, P. Energy Transfer Induced by Carbon Quantum Dots in Porous Zinc Oxide Nanocomposite Films. J. Phys. Chem. C 2015, 119, 2837–2843. [Google Scholar] [CrossRef]
- Xu, J.; Sahu, S.; Cao, L.; Anilkumar, P.; Tackett, K.N.; Qian, H.; Bunker, C.E.; Guliants, E.A.; Parenzan, A.; Sun, Y.-P. Carbon nanoparticles as chromophores for photon harvesting and photoconversion. ChemPhysChem 2011, 12, 3604–3608. [Google Scholar] [CrossRef] [PubMed]
- Posudievsky, O.Y.; Khazieieva, O.A.; Koshechko, V.G.; Pokhodenko, V.D. Preparation of graphene oxide by solvent-free mechanochemical oxidation of graphite. J. Mater. Chem. 2012, 22, 12465–12467. [Google Scholar] [CrossRef]
- Nguyen, V.; Yan, L.; Si, J.; Hou, X. Femtosecond laser-assisted synthesis of highly photoluminescent carbon nanodots for Fe3+detection with high sensitivity and selectivity. Opt. Mater. Express 2016, 6, 312–320. [Google Scholar] [CrossRef] [Green Version]
- Pan, M.; Xie, X.; Liu, K.; Yang, J.; Hong, L.; Wang, S. Fluorescent Carbon Quantum Dots—Synthesis, Functionalization and Sensing Application in Food Analysis. Nanomaterials 2020, 10, 930. [Google Scholar] [CrossRef]
- Liu, L.; Mi, Z.; Hu, Q.; Li, C.; Li, X.; Feng, F. Green synthesis of fluorescent carbon dots as an effective fluorescence probe for morin detection. Anal. Methods 2019, 11, 353–358. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, N.C.; Khan, S.; Tiwari, S.; Chaudhary, A.; Nandi, C.K. Paper strip based and live cell ultrasensitive lead sensor using carbon dots synthesized from biological media. Sens. Actuators B Chem. 2016, 232, 107–114. [Google Scholar] [CrossRef]
- Wang, H.; Ning, G.; He, X.; Ma, X.; Yang, F.; Xu, Z.; Li, Y. Carbon quantum dots derived by direct carbonization of carbonaceous microcrystals in mesophase pitch. Nanoscale 2018, 10, 21492–21498. [Google Scholar] [CrossRef]
- Ma, C.-B.; Zhu, Z.-T.; Wang, H.-X.; Huang, X.; Zhang, X.; Qi, X.; Zhang, H.-L.; Zhu, Y.; Deng, X.; Peng, Y.; et al. General solid-state synthesis of chemically-doped fluorescent graphene quantum dots for bioimaging and optoelectronic applications. Nanoscale 2015, 7, 10162–10169. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ding, Y.; Deng, Z.; Li, Z. Rational design of ternary NiS/CQDs/ZnIn2S4 nanocomposites as efficient noble-metal-free photocatalyst for hydrogen evolution under visible light. Chin. J. Catal. 2019, 40, 335–342. [Google Scholar] [CrossRef]
- Shen, T.; Wang, Q.; Guo, Z.; Kuang, J.; Cao, W. Hydrothermal synthesis of carbon quantum dots using different precursors and their combination with TiO2 for enhanced photocatalytic activity. Ceram. Int. 2018, 44, 11828–11834. [Google Scholar] [CrossRef]
- Zhao, C.; Jiao, Y.; Hua, J.; Yang, J.; Yang, Y. Hydrothermal synthesis of nitrogen-doped carbon quantum dots as fluorescent probes for the detection of dopamine. J. Fluoresc. 2018, 28, 269–276. [Google Scholar] [CrossRef]
- Nabiyouni, G.; Ghanbari, D. Hydrothermal Synthesis of Magnetic and Photoluminescence CuFe2O4-carbon dots nanocomposite as a sensor for detecting of Hg(II) ions. J. Nanostruct. 2020, 10, 760–768. [Google Scholar]
- Zhang, B.; Liu, C.-Y.; Liu, Y. A novel one-step approach to synthesize fluorescent carbon nanoparticles. Eur. J. Inorg. Chem. 2010, 28, 4411–4414. [Google Scholar] [CrossRef]
- Wang, X.; Yang, P.; Feng, Q.; Meng, T.; Wei, J.; Xu, C.; Han, J. Green preparation of fluorescent carbon quantum dots from cyanobacteria for biological imaging. Polymers 2019, 11, 616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Cui, Y.H.; Li, T.T.; Diao, H.P.; Wei, S.Y.; Li, L.H.; Chang, H.H.; Zhang, B.; Wei, W.L. Green and facile synthesis of highly photoluminescent nitrogen-doped carbon dots for sensors and cell imaging. Chem. Lett. 2018, 47, 421–424. [Google Scholar] [CrossRef]
- Das, R.K.; Kar, J.P.; Mohapatra, S. Enhanced photodegradation of organic pollutants by carbon quantum dot (CQD) deposited Fe3O4@ mTiO2 nano-pom-pom balls. Ind. Eng. Chem. 2016, 55, 5902–5910. [Google Scholar] [CrossRef]
- Aghamali, A.; Khosravi, M.; Hamishehkar, H.; Modirshahla, N.; Behnajady, M.A. Preparation of novel high performance recoverable and natural sunlight-driven nanocomposite photocatalyst of Fe3O4/C/TiO2/N-CQDs. Mater. Sci. Semicond. Process. 2018, 87, 142–154. [Google Scholar] [CrossRef]
- He, X.; Han, Y.; Luo, X.; Yang, W.; Li, C.; Tang, W.; Yue, T.; Li, Z. Terbium (III)-referenced N-doped carbon dots for ratiometric fluorescent sensing of mercury (II) in seafood. Food Chem. 2020, 320, 126624. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, X.; Li, Q.; Shi, Y.; Zhai, X.; Xu, Y.; Li, Z.; Huang, X.; Wang, X.; Shi, J.; et al. Copper nanoclusters@nitrogen-doped carbon quantum dots-based ratiometric fluorescence probe for lead (II) ions detection in porphyra. Food Chem. 2020, 320, 126623. [Google Scholar] [CrossRef] [PubMed]
- Al-Enizi, A.M.; Ubaidullah, M.; Kumar, D. Carbon quantum dots (CQDs)/Ce doped NiO nanocomposite for high performance supercapacitor. Mater. Today Commun. 2021, 27, 102340. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, X.; Li, Y.; Wang, Z.; Yang, F.; Yang, X. Microwave synthesis of fluorescent carbon nanoparticles with electrochemiluminescence properties. Chem. Commun. 2009, 1, 5118–5120. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, D.; Algarra, M.; Tarelho, L.A.C.; Frade, J.; Franco, A.; De Miguel, G.; Luque, R. Catalyzed microwave-assisted preparation of carbon quantum dots from lignocellulosic residues. ACS Sustain. Chem. Eng. 2018, 6, 7200–7205. [Google Scholar] [CrossRef]
- Choi, Y.; Thongsai, N.; Chae, A.; Jo, S.; Bi Kang, E.; Paoprasert, P.; Young Park, S.; In, I. Microwave-assisted synthesis of luminescent and biocompatible lysine-based carbon quantum dots. J. Ind. Eng. Chem. 2017, 47, 329–335. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, Z.; Chen, M.; Chen, W.; Zhou, X. Microwave-assisted synthesis of xylan-derived carbon quantum dots for tetracycline sensing. Opt. Mater. 2018, 85, 329–336. [Google Scholar] [CrossRef]
- Feng, J.; Zhao, X.; Bian, W.; Tang, X. Microwave-assisted synthesis of nitrogen-rich carbon dots as effective fluorescent probe for sensitive detection of Ag+. Mater. Chem. Front. 2019, 3, 2751–2758. [Google Scholar] [CrossRef]
- Kumar Mondal, T.; Kumar Ghorai, U.; Saha, S.K. Dual-emissive carbon quantum dot-Tb nanocomposite as a fluorescent indicator for a highly selective visual detection of Hg (II) in water. ACS Omega 2018, 3, 11439–11446. [Google Scholar] [CrossRef]
- Sharma, G.; Bhogal, S.; Naushad, M.; Kumar, A.; Stadler, F.J. Microwave assisted fabrication of La/Cu/Zr/carbon dots trimetallic nanocomposites with their adsorptional vs photocatalytic efficiency for remediation of persistent organic pollutants. J. Photochem. Photobiol. A Chem. 2017, 347, 235–243. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, S.; Kansal, S. Synthesis of ZnS/CQDs nanocomposite and its application as a photocatalyst for the degradation of an anionic dye. Superlattices Microstruct. 2016, 98, 86–95. [Google Scholar] [CrossRef]
- Liu, T.; Xue Dong, J.; Gang Liu, S.; Li, N.; Min Lin, S.; Zhu Fan, Y.; Lie Lei, J.; Qun Luo, H.; Bing Li, N. Carbon quantum dots prepared with polyethyleneimine as both reducing agent and stabilizer for synthesis of Ag/CQDs composite for Hg2+ ions detection. J. Hazard. Mater. 2017, 322, 430–436. [Google Scholar] [CrossRef]
- Briscoe, J.; Marinovic, A.; Sevilla, M.; Dunn, S.; Titirici, M. Biomass-derived carbon quantum dot sensitizers for solid-state nanostructured solar cells. Angew. Chem. 2015, 54, 4463–4468. [Google Scholar] [CrossRef]
- Arvand, M.; Hemmati, S. Magnetic nanoparticles embedded with graphene quantum dots and multiwalled carbon nanotubes as a sensing platform for electrochemical detection of progesterone. Sens. Actuators B Chem. 2017, 238, 346–356. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, R.; Singh, D.P.; Savu, R.; Moshkalev, S.A. Progress in microwave-assisted synthesis of quantum dots (graphene/carbon/semiconducting) for bioapplications: A review. Mater. Today Chem. 2019, 12, 282–314. [Google Scholar] [CrossRef]
- Xie, J.-D.; Lai, G.-W.; Mahmudul Huq, M. Hydrothermal route to graphene quantum dots: Effects of precursor and temperature. Diam. Relat. Mater. 2017, 79, 112–118. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, J.; Qing, Y.; Fan, C.; Wang, L.; Huang, Y.; Sheng, R.; Guo, Y.; Wang, T.; Pan, Y.; et al. Construction of hierarchical porous carbon nanosheets from template-assisted assembly of coal-based graphene quantum dots for high performance supercapacitor electrodes. Mater. Today Energy 2017, 6, 36–45. [Google Scholar] [CrossRef]
- Chen, A.; Zhao, C.; Yu, Y.; Yang, J. Graphene quantum dots derived from carbon fibers for oxidation of dopamine. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2016, 31, 1294–1297. [Google Scholar] [CrossRef]
- Huang, H.; Yang, S.; Li, Q.; Yang, Y.; Wang, G.; You, X.; Mao, B.; Wang, H.; Ma, Y.; He, P.; et al. Electrochemical cutting in weak aqueous electrolytes: The strategy for efficient and controllable preparation of graphene quantum dots. Langmuir 2018, 34, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, C.; Du, P.; Feng, X.; Wu, P.; Cai, C. Electrolyzing synthesis of boron-doped graphene quantum dots for fluorescence determination of Fe3+ions in water samples. Talanta 2017, 164, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Shinde, D.B.; Pillai, V.K. Electrochemical preparation of luminescent graphene quantum dots from multiwalled carbon nanotubes. Chem. Eur. J. 2012, 18, 12522–12528. [Google Scholar] [CrossRef]
- He, M.; Guo, X.; Huang, J.; Shen, H.; Zeng, Q.; Wang, L. Mass production of tunable multicolor graphene quantum dots from an energy resource of coke by a one-step electrochemical exfoliation. Carbon 2018, 140, 508–520. [Google Scholar] [CrossRef]
- Liu, F.; Sun, Y.; Zheng, Y.; Tang, N.; Li, M.; Zhong, W.; Du, Y. Gram-scale synthesis of high-purity graphene quantum dots with multicolor photoluminescence. RSC Adv. 2015, 5, 103428–103432. [Google Scholar] [CrossRef]
- Lu, Q.; Wu, C.; Liu, D.; Wang, H.; Su, W.; Li, H.; Zhang, Y.; Yao, S. A facile and simple method for synthesis of graphene oxide quantum dots from black carbon. Green Chem. 2017, 19, 900–904. [Google Scholar] [CrossRef]
- Peng, J.; Gao, W.; Kumar Gupta, B.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene Quantum Dots Derived from Carbon Fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef]
- Chen, W.; Li, F.; Wu, C.; Guo, T. Optical properties of fluorescent zigzag graphene quantum dots derived from multi-walled carbon nanotubes. Appl. Phys. Lett. 2014, 104, 063109. [Google Scholar] [CrossRef]
- Kiang Chua, C.; Sofer, Z.; Šimek, P.; Jankovský, O.; Klímová, K.; Bakardjieva, S.; HrdlickovaKuckova, S.; Pumera, M. Synthesis of strongly fluorescent graphene quantum dots by cage-opening buckminsterfullerene. ACS Nano 2015, 9, 2548–2555. [Google Scholar] [CrossRef] [PubMed]
- Castaneda-Serna, H.U.; Calderon-Dominguez, G.; Garcia-Borquez, A.; Salgado-Cruz, M.; Rebollo, R.R.F. Structural and luminescent properties of CQDs produced by microwave andconventional hydrothermal methods using pelagic Sargassum ascarbon source. Opt. Mater. 2022, 126, 112156. [Google Scholar] [CrossRef]
- Liang, Q.; Ma, W.; Shi, Y.; Li, Z.; Yang, X. Easy synthesis of highly fluorescent carbon quantum dots from gelatin and their luminescent properties and applications. Carbon 2013, 60, 421–428. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Wu, B.; Li, Z.; Wang, S.; Liu, Y.; Pan, D.; Wu, M. Facile synthesis of fluorescent graphene quantum dots from coffee grounds for bioimaging and sensing. Chem. Eng. J. 2016, 300, 75–82. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, J.; Li, Z. Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Sun, T.; Niu, A.; Tang, Y.-M.; Deng, S.; Luo, W.; Xu, Q.; Wei, D.; Pei, D.-S. Perturbation effect of reduced graphene oxide quantum dots (rGOQDs) on aryl hydrocarbon receptor (AhR) pathway in zebrafish. Biomaterials 2017, 133, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, J.; Zhang, X.; Li, N.; Liu, B.; Li, Y.; Wang, Y.; Wang, W.; Li, Y.; Zhang, L.; et al. Large-scale and controllable synthesis of graphene quantum dots from rice husk biomass: A comprehensive utilization strategy. ACS Appl. Mater. Interfaces 2016, 8, 1434–1439. [Google Scholar] [CrossRef]
- Roy, P.; Prakash Periasamy, A.; Chuang, C.; Liou, Y.-R.; Chen, Y.-F.; Joly, J.; Liang, C.-T.; Chang, H.-T. Plant leaf-derived graphene quantum dots and applications for white LEDs. New J. Chem. 2014, 38, 4946–4951. [Google Scholar] [CrossRef]
- Liu, Z.; Mo, Z.; Niu, X.; Yang, X.; Jiang, Y.; Zhao, P.; Liu, N.; Guo, R. Highly sensitive fluorescence sensor for mercury (II) based on boron-and nitrogen-co-doped graphene quantum dots. J. Colloid Interface Sci. 2020, 566, 357–368. [Google Scholar] [CrossRef]
- Pan, D.; Jiao, J.; Li, Z.; Guo, Y.; Feng, C.; Liu, Y.; Wang, L.; Wu, M. Efficient Separation of Electron–Hole Pairs in Graphene Quantum Dots by TiO2 Heterojunctions for Dye Degradation. ACS Sustain. Chem. Eng. 2015, 3, 2405–2413. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Z.; Gao, Y.; Chen, L.; Lu, M.; Jiao, Z.; Jiang, Y.; Ding, Y.; Cheng, L. Hydrothermal synthesis of layer-controlled MoS2/graphene composite aerogels for lithium-ion battery anode materials. Appl. Surf. Sci. 2016, 390, 209–215. [Google Scholar] [CrossRef]
- Luo, Y.; Li, M.; Sun, L.; Xu, Y.; Hu, G.; Tang, T.; Wen, J.; Li, X. Tuning the photoluminescence of graphene quantum dots by co-doping of nitrogen and sulfur. J. Nanopart. Res. 2017, 19, 363. [Google Scholar] [CrossRef]
- Fang, X.; Ding, J.; Yuan, N.; Sun, P.; Lv, M.; Ding, G.; Zhu, C. Graphene quantum dot incorporated perovskite films: Passivating grain boundaries and facilitating electron extraction. Phys. Chem. Chem. Phys. 2017, 19, 6057–6063. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Cao, X.; Lin, J.; Jiang, H.; Li, X.; Seng Teng, K.; Man Luk, C.; Zeng, S.; Hao, J.; et al. Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots. ACS Nano 2012, 6, 5102–5110. [Google Scholar] [CrossRef]
- Kop Alves, A.; Shuh Frantz, A.C.; Amorim Berutti, F. Microwave-assisted oleothermal synthesis of graphene-TiO2quantum dots for photoelectrochemical oxygen evolution reaction. FlatChem 2018, 12, 26–34. [Google Scholar] [CrossRef]
- Fresco-Cala, B.; Soriano, M.L.; Sciortino, A.; Cannas, M.; Messina, F.; Cardenas, S. One-pot synthesis of graphene quantum dots and simultaneous nanostructured self-assembly via a novel microwave-assisted method: Impact on triazine removal and efficiency monitoring. RSC Adv. 2018, 8, 29939–29946. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-L.; Ji, J.; Fei, R.; Wang, C.-Z.; Lu, Q.; Zhang, J.-R.; Jiang, L.-P.; Zhu, J.-J. A facile microwave avenue to electrochemiluminescent two-color graphene quantum dots. Adv. Funct. Mater. 2012, 22, 2971–2979. [Google Scholar] [CrossRef]
- Murugesan, B.; Sonamuthu, J.; Pandiyan, N.; Pandi, B.; Samayanan, S.; Mahalingam, S. Photoluminescent reduced graphene oxide quantum dots from latex of Calotropis gigantea for metal sensing, radical scavenging, cytotoxicity, and bioimaging in Artemia salina: A greener route. J. Photochem. Photobiol. B 2018, 178, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Li, M.; Xiao, J.; Liu, C.; Li, Z.; Xie, Y.; Ning, P.; Cao, H.; Zhang, Y. Novel oxidative cutting graphene oxide to graphene quantum dots for electrochemical sensing application. Mater. Today Commun. 2016, 8, 127–133. [Google Scholar] [CrossRef]
- Gao, H.; Xue, C.; Hu, G.; Zhu, K. Production of graphene quantum dots by ultrasound-assisted exfoliation in supercritical CO2/H2O medium. Ultrason. Sonochem. 2017, 37, 120–127. [Google Scholar] [CrossRef]
- Hyun Kang, S.; Mhin, S.; Han, H.; Min Kim, K.; Jones, J.L.; Ryu, J.H.; Seop Kang, J.; Hee Kim, S.; Bo Shim, K. Ultrafast method for selective design of graphene quantum dots with highly efficient blue emission. Sci. Rep. 2016, 6, 38423. [Google Scholar] [CrossRef] [Green Version]
- Bayat, A.; Saievar-Iranizad, E. Synthesis of green-photoluminescent single layer graphene quantum dots: Determination of HOMO and LUMO energy states. J. Lumin. 2017, 192, 180–183. [Google Scholar] [CrossRef]
- Teymourinia, H.; Salavati-Niasari, M.; Amiri, O.; Safardoust-Hojaghan, H. Synthesis of graphene quantum dots from corn powder and their application in reduce charge recombination and increase free charge carriers. J. Mol. Liq. 2017, 242, 447–455. [Google Scholar] [CrossRef]
- Hong, G.-L.; Zhao, H.-L.; Deng, H.-H.; Yang, H.-J.; Peng, H.-P.; Liu, Y.-H.; Chen, W. Fabrication of ultra-small monolayer graphene quantum dots by pyrolysis of trisodium citrate for fluorescent cell imaging. Int. J. Nanomed. 2018, 13, 4807–4815. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.T.; Gonzalez-Rodriguez, R.; Ryan, C.; Faerber, N.; Coffer, J.L.; Naumov, A.V. Photo-and electroluminescence from nitrogen-doped and nitrogen-sulfurcodoped graphene quantum dots. Adv. Funct. Mater. 2018, 28, 1804337. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Zhao, Y. An electrochemical avenue to green-luminescent graphene quantum dots as potential electron- acceptors for photovoltaics. J. Adv. Mater. 2011, 23, 776–780. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Xu, T.; Liao, H.; Yao, C.; Liu, Y.; Li, Z.; Chen, Z.; Pan, D.; Sun, L.; et al. Gram-scale synthesis of single-crystalline graphene quantum dots with superior optical properties. Nat. Commun. 2014, 5, 5357. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Chen, C.; Zheng, X.; Gao, L.; Cui, Z.; Yang, H.; Guo, C.; Chi, Y.; Ming Li, C. One-step and high yield simultaneous preparation of single-and multi-layer graphene quantum dots from CX-72 carbon black. J. Mater. Chem. 2012, 22, 8764–8766. [Google Scholar] [CrossRef]
- Kadian, S.; Manik, G. Sulfur doped graphene quantum dots as a potential sensitive fluorescent probe for the detection of quercetin. Food Chem. 2020, 317, 126457. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Tao, Y.; Ma, J.; Kong, Y. Well-dispersed chitosan-graphene quantum dots nanocomposites for electrochemical sensing platform. J. Electrochem. Soc. 2015, 162, H884–H889. [Google Scholar] [CrossRef]
- Bu, X.; Yang, S.; Bu, Y.; He, P.; Yang, Y.; Wang, G.; Li, H.; Wang, P.; Wang, X.; Ding, G.; et al. Highly Active Black TiO2/N-doped Graphene Quantum Dots Nanocomposites for Sunlight Driven Photocatalytic Sewage Treatment. ChemistrySelect 2018, 3, 201–206. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Yu, S.; Xie, Z.; Chen, Y.; Zheng, K.; Mossin, S.; Lin, W.; Meng, J.; Pullerits, T.; et al. Defect State Assisted Z-scheme Charge Recombination in Bi2O2CO3/Graphene Quantum Dot Composites for Photocatalytic Oxidation of NO. ACS Appl. Nano Mater. 2020, 3, 772–781. [Google Scholar] [CrossRef]
- Ju, J.; Zhang, R.; He, S.; Chen, W. Nitrogen-doped graphene quantum dots-based fluorescent probe for the sensitive turn- on detection of glutathione and its cellular imaging. RSC Adv. 2014, 4, 52583–52589. [Google Scholar] [CrossRef]
- Joyti Deka, M.; Chowdhury, D. CVD assisted hydrophobic graphene quantum dots: Fluorescence sensor for aromatic amino acids. ChemistrySelect 2017, 2, 1999–2005. [Google Scholar] [CrossRef]
- Athika, M.; Prasath, A.; Duraisamy, E.; Devi, V.S.; Sharma, A.S.; Elumalai, P. Carbon-quantum dots derived from denatured milk for efficient chromium-ion sensing and supercapacitor applications. Mater. Lett. 2019, 241, 156–159. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, M.; Bhandari, B.; Yang, C.H. Food waste as a carbon source in carbon quantum dots technology and their applications in food safety detection. Trends Food Sci. Technol. 2020, 95, 86–96. [Google Scholar] [CrossRef]
- Joshi, P.N.; Mathias, A.; Mishra, A.; Mathias, A. Synthesis of ecofriendly fluorescent car- bon dots and their biomedical and environmental applications. Mater. Technol. 2018, 33, 672–680. [Google Scholar] [CrossRef]
- Sabet, M.; Mahdavi, K. Green synthesis of high photoluminescence nitrogen-doped carbon quantum dots from grass via a simple hydrothermal method for removing organic and inorganic water pollutions. Appl. Surf. Sci. 2019, 463, 283–291. [Google Scholar] [CrossRef]
- Ramar, V.; Moothattu, S.; Balasubramanian, K. Metal free, sunlight and white light based photocatalysis using carbon quantum dots from Citrus grandis: A green way to remove pollution. Sol. Energy 2018, 169, 120–127. [Google Scholar] [CrossRef]
- Xue, M.Y.; Zou, M.B.; Zhao, J.J.; Zhan, Z.H.; Zhao, S.L. Green preparation of fluorescent carbon dots from lychee seeds and their application for the selective detection of methylene blue and imaging in living cells. J. Mater. Chem. B 2015, 3, 6783–6789. [Google Scholar] [CrossRef]
- Xue, M.Y.; Zhan, Z.H.; Zou, M.B.; Zhang, L.L.; Zhao, S.L. Green synthesis of stable and biocompatible fluorescent carbon dots from peanut shells for multicolor living cell imaging. New J. Chem. 2016, 40, 1698–1703. [Google Scholar] [CrossRef]
- Bankoti, K.; Rameshbabu, A.P.; Datta, S.; Das, B.; Mitra, A.; Dhara, S. Onion derived carbon nanodots for live cell imaging and accelerated skin wound healing. J. Mater. Chem. B 2017, 5, 6579–6592. [Google Scholar] [CrossRef]
- Pandiyan, S.; Arumugam, L.; Srirengan, S.P.; Pitchan, R.; Sevugan, P.; Kannan, K.; Pitchan, G.; Hegde, T.A.; Gandhirajan, V. Biocompatible Carbon Quantum Dots Derived from Sugarcane Industrial Wastes for Effective Nonlinear Optical Behavior and Antimicrobial Activity Applications. ACS Omega 2020, 5, 30363–30372. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Q.; Zhou, Z.; Zhao, S.; Zhong, S.; Xu, L.; Gao, Y.; Cui, X. Green synthesis of carbon quantum dots from corn stalk shell by hydrothermal approach in near-critical water and applications in detecting and bioimaging. Microchem. J. 2021, 166, 106250. [Google Scholar] [CrossRef]
- Hui, K.C.; Ang, W.L.; Sambudi, N.S. Nitrogen and bismuth-doped rice husk-derived carbon quantum dots for dye degradation and heavy metal removal. J. Photochem. Photobiol. A Chem. 2021, 418, 113411. [Google Scholar]
- Baweja, H.; Jeet, K. Economical and green synthesis of graphene and carbon quantum dots from agriculture waste. Mater. Res. Express 2019, 6, 0850g8. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Q.; Chen, D.; Liu, Z.; Zheng, X.; Xu, A.; Yang, S.; Ding, G. Facile and Highly Effective Synthesis of Controllable Lattice Sulfur-Doped Graphene Quantum Dots via Hydrothermal Treatment of Durian. ACS Appl. Mater. Interfaces 2018, 10, 5750–5759. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Z.; Liu, J.; Peng, Y.; Yu, X.; Wang, W. One-Pot Facile Synthesis of Graphene Quantum Dots from Rice Husks for Fe3+ Sensing. Ind. Eng. Chem. Res. 2018, 57, 9144–9150. [Google Scholar] [CrossRef]
- Ahmed, D.S.; Mohammed, M.; Majeed, S.M. Green Synthesis of Eco-Friendly Graphene Quantum Dots for Highly Efficient Perovskite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 10863–10871. [Google Scholar] [CrossRef]
- Chen, X.; Gong, F.; Cao, Z.; Zou, W.; Gu, T. Highly cysteine-selective fluorescent nanoprobes based on ultrabright and directly synthesized carbon quantum dots. Anal. Bioanal. Chem. 2018, 410, 2961–2970. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, C.; Lu, Y.; Chen, X.; Yuan, H.; Wei, G.; Ye, G.; Chen, J. Fluorescence Sensor Array based on Amino Acid Derived Carbon Dots for Pattern-based Detection of Toxic Metal Ions. Sens. Actuators B Chem. 2016, 241, 1324–1330. [Google Scholar] [CrossRef]
- Gao, G.; Jiang, Y.-W.; Jia, H.-R.; Yang, J.; Wu, F.-G. On-off-on fluorescent nanosensor for Fe3+ detection and cancer/normal cell differentiation via silicon-doped carbon quantum dots. Carbon 2018, 134, 232–243. [Google Scholar] [CrossRef]
- Li, C.; Liu, W.; Ren, Y.; Sun, X.; Pan, W.; Wang, J. The selectivity of the carboxylate groups terminated carbon dots switched by buffer solutions for the detection of multi-metal ions. Sens. Actuators B Chem. 2017, 240, 941–948. [Google Scholar] [CrossRef]
- Vinod Kumar, V.; Raman, T.; Philip Anthony, S. Fluorescent carbon quantum dots chemosensor for selective turn-on sensing of Zn2+ and turn-off sensing of Pb2+ in aqueous medium and Zebra fish egg. New J. Chem. 2017, 41, 15157–15164. [Google Scholar] [CrossRef]
- Yan, X.; Song, Y.; Zhu, C.; Li, H.; Du, D.; Su, X.; Lin, Y. MnO2nanosheet-carbon dots sensing platform for sensitive detection of organophosphorus pesticides. Anal. Chem. 2018, 90, 2618–2624. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, H.; Lin, J.-M. Peroxide induced ultra-weak chemiluminescence and its application in analytical chemistry. Analyst 2013, 138, 5182–5193. [Google Scholar] [CrossRef] [PubMed]
- Ali Shah, S.N.; Dou, X.; Khan, M.; Uchiyama, K.; Lin, J.-M. N-doped carbon dots/H2O2 chemiluminescence system for selective detection of Fe2+ ion in environmental samples. Talanta 2019, 196, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Manzoori, J.L.; Hallaj, T.; Azizi, N. Sulfur and nitrogen co-doped carbon quantum dots as the chemiluminescence probe for detection of Cu2+ ions. J. Lumin. 2017, 182, 246–251. [Google Scholar] [CrossRef]
- Nakano, K.; Honda, T.; Yamasaki, K.; Tanaka, Y.; Taniguchi, K.; Ishimatsu, R.; Imato, T. Carbon quantum dots as fluorescent component in peroxyoxalate chemiluminescence for hydrogen peroxide determination. Bull. Chem. Soc. Jpn. 2018, 91, 1128–1130. [Google Scholar] [CrossRef]
- Amjadi, M.; Hallaj, T. Dramatic enhancement effect of carbon quantum dots on the chemiluminescence of Ru (bpy)32+–Ce (IV) reaction and application to the determination of 4-nitrophenol. J. Lumin. 2016, 171, 202–207. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Wei, W.; Qu, X. Recent advances in graphene quantum dots for sensing. Mater. Today 2013, 16, 433–442. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, B.; Gu, S.; Lv, S.; Zhou, Y.; Liu, B. Facile synthesis of fluorescent carbon nanodots from cornstalk and their application as a sensing platform for detection of Cu2+ ions. Sci. Adv. Mater. 2017, 9, 901–906. [Google Scholar] [CrossRef]
- Dai, G.; Wang, J.; Zhao, Y.; Zhu, Y.; Ma, X.; Mei, T.; Li, J.; Ma, P.; Wang, X. Dual-mode high sensitive detection of Fe(III) ions via fluorescent photonic crystal films based on co-assembly of silica colloids and carbon dots. Sci. Adv. Mater. 2017, 9, 873–880. [Google Scholar] [CrossRef]
- Bin Chen, B.; Xi Liu, Z.; Yan Zou, H.; Zhi Huang, C. Highly selective detection of 2,4,6-trinitrophenol by using newly developed terbium-doped blue carbon dots. Analyst 2016, 141, 2676–2681. [Google Scholar] [CrossRef]
- Li Liu, M.; Bin Chen, B.; Yang, T.; Wang, J.; Dong Liu, X.; Zhi Huang, C. One-pot carbonization synthesis of europium-doped carbon quantum dots for highly selective detection of tetracycline. Methods Appl. Fluoresc. 2017, 5, 015003. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Wang, Y.; Xiao, S.; Wang, H.; Wang, J.-H.; Feng, L. Rapid detection of Cr(VI) ions based on cobalt(II)-doped carbon dots. Biosens. Bioelectron. 2017, 87, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wei, J.; Wang, J.; Liu, Y.; Li, N.; Chen, Y.; Gao, C.; Zhang, W.; Sreenivasan Sreeprased, T. Facile synthesis of copper doped carbon dots and their application as a “turn-off” fluorescent probe in the detection of Fe3þ ions. RSC Adv. 2016, 6, 28745–28750. [Google Scholar] [CrossRef]
- Costas-Mora, I.; Romero, V.; Lavilla, I.; Bendicho, C. In Situ Building of a Nanoprobe Based on Fluorescent Carbon Dots for Methylmercury Detection. Anal. Chem. 2014, 86, 4536–4543. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Li, S.; Zhuang, L.; Tang, J. L-Tryptophan-capped carbon quantum dots for the sensitive and selective fluorescence detection of mercury ion in aqueous solution. J. Nanopart. Res. 2016, 18, 202. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Q.; Long, Y.; Cheng, Z.; Chen, S.; Zheng, H.; Huang, Y. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem. Commun. 2011, 47, 6695–6697. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Ge, S.; Wang, S.; Yan, M.; Zang, D.; Yu, J. Facile and sensitive paper-based chemiluminescence DNA biosensor using carbon dots dotted nanoporous gold signal amplification label. Anal. Methods 2013, 5, 1328–1336. [Google Scholar] [CrossRef]
- Kumar, A.; Chowdhuri, A.R.; Laha, D.; Mahto, T.K.; Karmakar, P.; Sahu, S.K. Green synthesis of carbon dots from Ocimumsanctum for effective fluorescent sensing of Pb2+ ions and live cell imaging. Sens. Actuators B Chem. 2017, 242, 679–686. [Google Scholar] [CrossRef]
- KaanKoc, O.; Uzer, A.; Apak, R. High Quantum Yield Nitrogen-Doped Carbon Quantum Dot-Based Fluorescent Probes for Selective Sensing of 2,4,6-Trinitrotoluene. ACS Appl. Nano Mater. 2022, 5, 5868–5881. [Google Scholar]
- Ashrafi Tafreshi, F.; Fatahi, Z.; Fatemeh Ghasemi, S.; Taherian, A.; Esfandiari, N. Ultrasensitive fluorescent detection of pesticides in real sample by using green carbon dots. PLoS ONE 2020, 15, e0230646. [Google Scholar] [CrossRef]
- Khaledian, S.; Noroozi-Aghideh, A.; Kahrizi, D.; Moradi, S.; Abdoli, M.; Haji Ghasemalian, A.; FoadHeidari, M. Rapid detection of diazinon as an organophosphorus poison in real samples using fluorescence carbon dots. Inorg. Chem. Commun. 2021, 130, 108676. [Google Scholar] [CrossRef]
- Ali Kamyabi, M.; Moharramnezhad, M. A novel cathodic electrochemiluminescent sensor based on CuS/carbon quantum dots/g-C3N4 nanosheets and boron nitride quantum dots for the sensitive detection of organophosphate pesticide. Microchem. J. 2022, 179, 107421. [Google Scholar] [CrossRef]
- Duan, L.; Du, X.; Zhao, H.; Sun, Y.; Liu, W. Sensitive and selective sensing system of metallothioneins based on Carbon Quantum Dots and gold nanoparticles. Anal. Chim. Acta 2020, 1125, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fang, G.; Wang, X.; Zhang, F.; Liu, J.; Zheng, W.; Wang, S. Electrochemiluminescent graphene quantum dots enhanced by MoS2 as sensing platform: A novel molecularly imprinted electrochemiluminescence sensor for 2-methyl-4-chlorophenoxyacetic acid assay. Electrochim. Acta 2017, 228, 107–113. [Google Scholar] [CrossRef]
- Sammi, H.; Kukkar, D.; Singh, J.; Kukkar, P.; Kaur, R.; Kaur, H.; Rawat, M.; Singh, G.; Kim, K.-H. Serendipity in solution–GQDs zeolitic imidazole frameworks nanocomposites for highly sensitive detection of sulfide ions. Sens. Actuators B Chem. 2017, 255, 3047–3056. [Google Scholar] [CrossRef]
- Li, J.; Wang, N.; Tran, T.T.; Huang, C.; Chen, L.; Yuan, L.; Zhou, L.; Shen, R.; Cai, Q. Electrogenerated chemiluminescence detection of trace level pentachlorophenol using carbon quantum dots. Analyst 2013, 138, 2038–2043. [Google Scholar] [CrossRef]
- Zhang, X.; Liao, X.; Hou, Y.; Jia, B.; Fu, L.; Jia, M.; Zhou, L.; Lu, J.; Kong, W. Recent advances in synthesis and modification of carbon dots for optical sensing of pesticides. J. Hazard. Mater. 2022, 422, 126881. [Google Scholar] [CrossRef]
- Qin, X.; Lu, W.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Microwave-assisted rapid green synthesis of photoluminescent carbon nanodots from flour and their applications for sensitive and selective detection of mercury(II) ions. Sens. Actuators B Chem. 2013, 184, 156–162. [Google Scholar] [CrossRef]
- Hou, Y.; Lu, Q.; Deng, J.; Li, H.; Zhang, Y. One-pot electrochemical synthesis of functionalized fluorescent carbon dots and their selective sensing for mercury ion. Anal. Chim. Acta 2015, 866, 69–74. [Google Scholar] [CrossRef]
- Gogoi, N.; Barooah, M.; Majumdar, G.; Chowdhury, D. Carbon dots rooted agarose hydrogel hybrid platform for optical detection and separation of heavy metal ions. ACS Appl. Mater. Interfaces 2015, 7, 3058–3067. [Google Scholar] [CrossRef]
- Lan, M.; Di, Y.; Zhu, X.; Ng, T.-W.; Xia, J.; Liu, W.; Meng, X.; Wang, P.; Lee, C.-S.; Zhang, W. A carbon dot-based fluorescence turn-on sensor for hydrogen peroxide with a photo-induced electron transfer mechanism. Chem. Commun. 2015, 51, 15574–15577. [Google Scholar] [CrossRef]
- Qin, T.; Wang, J.; Liu, Y.; Guo, S. Carbon Quantum Dots Based Chemosensor Array for Monitoring Multiple Metal Ions. Molecules 2022, 27, 3843. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhang, L.; Long, H.; Meng, M.; Liu, T.; Yin, Y.; Xi, R. A multianalyte fluorescent carbon dots sensing system constructed based on specific recognition of Fe(III) ions. RSC Adv. 2017, 7, 28637. [Google Scholar] [CrossRef]
- Faridbod, F.; Sanati, A.L. Graphene Quantum Dots in Electrochemical Sensors/Biosensors. Curr. Anal. Chem. 2018, 15, 103–123. [Google Scholar] [CrossRef]
- Tachi, S.; Morita, H.; Takahashi, M.; Okabayashi, Y.; Hosokai, T.; Sugai, T.; Kuwahara, S. Quantum Yield Enhancement in Graphene Quantum Dots via Esterification with Benzyl Alcohol. Sci. Rep. 2019, 9, 14115. [Google Scholar] [CrossRef]
- Pedrero, M.; Campuzano, S.; Pingarrón, J.M. Quantum dots as components of electrochemical sensing platforms for the detection of environmental and food pollutants: A review. J. AOAC Int. 2017, 100, 950–961. [Google Scholar] [CrossRef]
- Roushani, M.; Valipour, A. The potentiality of graphene quantum dots functionalized by nitrogen and thiol-doped (GQDs-N-S) to stabilize the antibodies in designing of human chorionic gonadotropin immunosensor. Nanochem. Res. 2019, 4, 20–26. [Google Scholar]
- Ma, F.; Li, C.-C.; Zhang, C.-Y. Development of quantum dot-based biosensors: Principles and applications. J. Mater. Chem. B 2018, 6, 6173–6190. [Google Scholar] [CrossRef]
- Xi, J.; Xie, C.; Zhang, Y.; Wang, L.; Xiao, J.; Duan, X.; Ren, J.; Xiao, F.; Wang, S. Pd nanoparticles decorated N-Doped graphene quantumdots@N-Dopedcarbon hollow nanospheres with high electrochemical sensing performance in cancer detection. ACS Appl. Mater. Interfaces 2016, 8, 22563–22573. [Google Scholar] [CrossRef]
- Jiang, D.; Du, X.; Qian, L.; Hao, N.; Wang, K. MoS2/nitrogen doped graphene hydrogels p-n heterojunction: Efficient charge transfer property for highly sensitive and selective photoelectrochemical analysis of chloramphenicol. Biosens. Bioelectron. 2018, 126, 463–469. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, X.; Deng, M.; Cao, Y.; Li, Y.; Zheng, H.; Li, F.; Yan, F.; Lan, T.; Shi, L.; et al. Nitrogen doped graphene quantum dots as a fluorescent probe for mercury(II) ions. Microchim. Acta 2019, 186, 140. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, X.; Xing, X.; Wang, Z.; Zou, T.; Wang, Z.; Zhao, R.; Wang, Y. One-pot synthesis of N-doped graphene quantum dots as highly sensitive fluorescent sensor for detection of mercury ions water solutions. Mater. Res. Express 2019, 6, 095615. [Google Scholar] [CrossRef]
- Lu, L.; Zhou, L.; Chen, J.; Yan, F.; Liu, J.; Dong, X.; Xi, F.; Chen, P. Nanochannel-Confined Graphene Quantum Dots for Ultrasensitive Electrochemical Analysis of Complex Samples. ACS Nano 2018, 12, 12673–12681. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.V.; Thankam Thomas, R.; Mohamed, A.P.; Pillai, S. Fluorescent turn-off sensor based on sulphur-doped graphene quantum dots in colloidal and film forms for the ultrasensitive detection of carbamate pesticides. Microchem. J. 2020, 157, 104971. [Google Scholar] [CrossRef]
- Suryawanshi, A.; Biswal, M.; Mhamane, D.; Gokhale, R.; Patil, S.; Guin, D.; Ogale, S. Large scale synthesis of graphene quantum dots (GQDs) from waste biomass and their use as an efficient and selective photoluminescence on–off–on probe for Ag+ ions. Nanoscale 2014, 6, 11664–11670. [Google Scholar] [CrossRef]
- Fan, L.; Hu, Y.; Wang, X.; Zhang, L.; Li, F.; Han, D.; Niu, L. Fluorescence resonance energy transfer quenching at the surface of graphene quantum dots for ultrasensitive detection of TNT. Talanta 2012, 101, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Shi, H.; He, X.; Wang, K.; He, D.; Yan, L.; Ye, X.; Tang, J.; Shangguan, J.; Luo, L. Masking agent-free and channel-switch-mode simultaneous sensing of Fe3+and Hg2+using dual-excitation graphene quantum dots. Analyst 2015, 140, 3925–3928. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Singh, S.; Bharwaj, S.K.; Kaur, G.; Khatri, M.; Deep, A.; Bhardwaj, N. Development of carbon quantum dot-based lateral flow immunoassay for sensitive detection of aflatoxin M1 in milk. Food Chem. 2022, 393, 13374. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Zhou, P.; Yang, W.; Tao, H.; Qiu, S.; Feng, C. A novel fluorescent aptasensor for ultrasensitive and selective detection of acetamiprid pesticide based on inner filter effect between gold nanoparticles and carbon dots. Analyst 2018, 143, 5151–5160. [Google Scholar] [CrossRef]
- Wu, M.; Fan, Y.; Li, J.; Lu, D.; Guo, Y.; Xie, L.; Wu, Y. Vinyl Phosphate-Functionalized, Magnetic, Molecularly-Imprinted Polymeric Microspheres’ Enrichment and Carbon Dots’ Fluorescence-Detection of Organophosphorus Pesticide Residues. Polymers 2019, 11, 1770. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hu, A. Carbon Quantum Dots. Synthesis, Properties and Applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Molaei, M.J. A review on nanostructured carbon quantum dots and their applications in biotechnology, sensors, and chemiluminescence. Talanta 2019, 196, 456–478. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Umar, A.; Sood, S.; Kumar Mehta, S.; Kumar Kansal, S. Photoluminescent C-dots: An overview on the recent development in the synthesis, physiochemical properties and potential applications. J. Alloys Compd. 2018, 748, 818–853. [Google Scholar] [CrossRef]













| Sr. No. | Sensing Platform | Analyte Detected | LOD | Detection Range | Reference |
|---|---|---|---|---|---|
| 1 | Cu-CQD | Fe3+ | 1 nM | 0.001–200 μM | [183] |
| 2 | Tb-CQD | Hg2+ | 168.8 ppb | 0.2–0.8 ppm | [99] |
| 3 | Ag-CQD | Hg2+ | 85 nM | 0.5–50 μM | [184] |
| 4 | L-Tryptophan-CQD | Hg2+ | 11 nM | _ | [102] |
| 5 | N-CQD-Tb | Hg2+ | 37 nM | 1–161.51 μM | [185] |
| 6 | CuNCs-CNQDs | Pb2+ | 0.0031 mg/L | 0.01–2.5 mg/L | [92] |
| 7 | NGQD | Hg2+ | 2.5 nM | 2.5–800 μM | [211] |
| 8 | N-GQD | Hg2+ | 0.45 nM | 1–1000 nM | [212] |
| 9 | CS-GQD/Bi modified GCE | Zn2+ | 8.84 μg/L | 50–450 μg/L | [146] |
| Cd2+ | 1.99 μg/L | ||||
| Pb2+ | 3.10 μg/L | ||||
| 10 | PEI-GQD | Fe3+, Cu2+ | 40 mg/L | 0–1 μM | [214] |
| Sr. No. | Sensing Platform | Analyte | LOD | Reference |
|---|---|---|---|---|
| 1. | CQDs | Diazinon, glyphosate, and amicarbazone | 0.25, 0.5 and 2 ng/mL | [190] |
| 2. | CQDs | Diazinon | 0.038 ± 0.01 µM | [191] |
| 3. | CuS/CQDs/g-C3N4 | Diazinon | 2.2 × 10−6 M | [192] |
| 4. | N,P-CQDs/Au NPs | Carbendazim | 0.002 µM | [193] |
| 5. | MoS2-GQDs | 2-methyl-4-chlorophenoxyacetic acid | 5.5 pmol/L | [194] |
| 6. | CQDs | pentachlorophenol | 1.3 × 10−12 g/L | [196] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, A.; Pandey, K.; Kaur, R.; Vashishat, N.; Kaur, M. Nanocomposites of Carbon Quantum Dots and Graphene Quantum Dots: Environmental Applications as Sensors. Chemosensors 2022, 10, 367. https://doi.org/10.3390/chemosensors10090367
Kaur A, Pandey K, Kaur R, Vashishat N, Kaur M. Nanocomposites of Carbon Quantum Dots and Graphene Quantum Dots: Environmental Applications as Sensors. Chemosensors. 2022; 10(9):367. https://doi.org/10.3390/chemosensors10090367
Chicago/Turabian StyleKaur, Ajaypal, Komal Pandey, Ramandeep Kaur, Nisha Vashishat, and Manpreet Kaur. 2022. "Nanocomposites of Carbon Quantum Dots and Graphene Quantum Dots: Environmental Applications as Sensors" Chemosensors 10, no. 9: 367. https://doi.org/10.3390/chemosensors10090367