Recovery of Citric Acid from Citrus Peels: Ultrasound-Assisted Extraction Optimized by Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Citrus Samples
2.2. Standards and Reagents
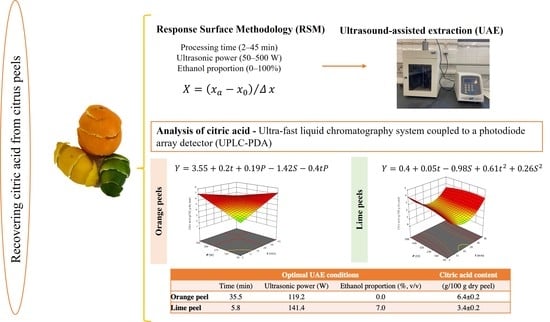
2.3. Experimental Design for Extraction Process Optimization
2.4. Ultrasound-Assisted Extraction (UAE)
2.5. Analysis of Citric Acid
2.6. Models Fitting and Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Usmani, Z.; Sharma, M.; Karpichev, Y.; Pandey, A.; Chandra Kuhad, R.; Bhat, R.; Punia, R.; Aghbashlo, M.; Tabatabaei, M.; Gupta, V.K. Advancement in valorization technologies to improve utilization of bio-based waste in bioeconomy context. Renew. Sustain. Energy Rev. 2020, 131, 109965. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Ligas, B.; Skrzypczak, D.; Mikula, K.; Izydorczyk, G.; Witek-Krowiak, A.; Moustakas, K.; Chojnacka, K. Biosorption as a method of biowaste valorization to feed additives: RSM optimization. Environ. Pollut. 2021, 268, 115937. [Google Scholar] [CrossRef] [PubMed]
- Poyraz, Ç.; Küçükyıldız, G.; Kırbaşlar, Ş.İ.; Ciğeroğlu, Z.; Şahin, S. Valorization of Citrus unshiu biowastes to value-added products: An optimization of ultrasound-assisted extraction method using response surface methodology and particle swarm optimization. Biomass Convers. Biorefin. 2021, 1–11. [Google Scholar] [CrossRef]
- Conceição, N.; Albuquerque, B.R.; Pereira, C.; Corrêa, R.C.G.; Lopes, C.B.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Ferreira, I.C.F.R. By-products of camu-camu [Myrciaria dubia (Kunth) McVaugh] as promising sources of bioactive high added-value food ingredients: Functionalization of yogurts. Molecules 2020, 25, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, S.; Shekher Giri, B.; Kumar Patel, A.; Sar, T.; Liu, H.; Chen, H.; Juneja, A.; Kumar, D.; Zhang, Z.; Kumar Awasthi, M.; et al. Resource recovery and biorefinery potential of apple orchard waste in the circular bioeconomy. Bioresour. Technol. 2021, 321, 124496. [Google Scholar] [CrossRef]
- Alemán-Ramirez, J.L.; Okoye, P.U.; Torres-Arellano, S.; Mejía-Lopez, M.; Sebastian, P.J. A review on bioenergetic applications of Leucaena leucocephala. Ind. Crops Prod. 2022, 182, 114847. [Google Scholar] [CrossRef]
- Carocho, M.; Barros, L.; Barreira, J.C.M.; Calhelha, R.C.; Soković, M.; Fernández-Ruiz, V.; Buelga, C.S.; Morales, P.; Ferreira, I.C.F.R. Basil as functional and preserving ingredient in “serra da Estrela” cheese. Food Chem. 2016, 207, 51–59. [Google Scholar] [CrossRef] [Green Version]
- EFSA. European Food Safety Authority. Directive 2008/98/EC of European Parliament and of the Council. Available online: https://eurlex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008L0098&from=EN (accessed on 1 June 2022).
- FDA. Use of the Term Natural on Food Labeling|FDA. 2018. Available online: https://www.fda.gov/food/food-labeling-nutrition/use-term-natural-food-labeling (accessed on 1 June 2022).
- FAO. Citrus Fruit. Fresh and processed-Statistical Bulletin 2016. Trade and Markets Division, 47, 2017. Available online: http://www.fao.org/3/a-i8092e.pdf (accessed on 1 June 2022).
- Rafiq, S.; Kaul, R.; Sofi, S.A.; Bashir, N.; Nazir, F.; Ahmad Nayik, G. Citrus peel as a source of functional ingredient: A review. J. Saudi Soc. Agric. Sci. 2018, 17, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Chavan, P.; Singh, A.K.; Kaur, G. Recent progress in the utilization of industrial waste and by-products of citrus fruits: A review. J. Food Process Eng. 2018, 41, e12895. [Google Scholar] [CrossRef]
- Farag, M.A.; Abib, B.; Ayad, L.; Khattab, A.R. Sweet and bitter oranges: An updated comparative review of their bioactives, nutrition, food quality, therapeutic merits and biowaste valorization practices. Food Chem. 2020, 331, 127306. [Google Scholar] [CrossRef] [PubMed]
- Ruviaro, A.R.; de Paula Menezes Barbosa, P.; Alexandre, E.C.; Justo, A.F.O.; Antunes, E.; Macedo, G.A. Aglycone-rich extracts from citrus by-products induced endothelium-independent relaxation in isolated arteries. Biocatal. Agric. Biotechnol. 2020, 23, 101481. [Google Scholar] [CrossRef]
- Mani-López, E.; García, H.S.; López-Malo, A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res. Int. 2012, 45, 713–721. [Google Scholar] [CrossRef]
- Behera, B.C.; Mishra, R.; Mohapatra, S. Microbial citric acid: Production, properties, application, and future perspectives. Food Front. 2021, 2, 62–76. [Google Scholar] [CrossRef]
- Sawant, O.; Mahale, S.; Ramchandran, V.; Nagaraj, G.; Bankar, A. Fungal Citric acid production using waste materials: A mini-review. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 821–828. [Google Scholar] [CrossRef]
- Ozdal, M.; Kurbanoglu, E.B. Citric Acid Production by Aspergillus niger from Agro-Industrial By-Products: Molasses and Chicken Feather Peptone. Waste Biomass Valorization 2019, 10, 631–640. [Google Scholar] [CrossRef]
- Fernández-Fernández, R.; López-Martínez, J.C.; Romero-González, R.; Martínez-Vidal, J.L.; Alarcón Flores, M.I.; Garrido Frenich, A. Simple LC-MS determination of citric and malic acids in fruits and vegetables. Chromatographia 2010, 72, 55–62. [Google Scholar] [CrossRef]
- Ayache, S.B.; Reis, F.S.; Dias, M.I.; Pereira, C.; Glamočlija, J.; Soković, M.; Saafi, E.B.; Ferreira, I.C.F.R.; Barros, L.; Achour, L. Chemical characterization of carob seeds (Ceratonia siliqua L.) and use of different extraction techniques to promote its bioactivity. Food Chem. 2021, 351, 129263. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- European Parliament Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009. Off. J. Eur. Union 2009, 140, 16–62.
- Dias, M.I.; Albiston, C.; Añibarro-Ortega, M.; Ferreira, I.C.F.R.; Pinela, J.; Barros, L. Sonoextraction of phenolic compounds and saponins from Aesculus hippocastanum seed kernels: Modeling and optimization. Ind. Crops Prod. 2022, 185, 115142. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized analysis of organic acids in edible mushrooms from Portugal by ultra fast liquid chromatography and photodiode array detection. Food Anal. Methods 2013, 6, 309–316. [Google Scholar] [CrossRef]
- Leichtweis, M.G.; Pereira, C.; Prieto, M.A.; Barreiro, M.F.; Beraldi, I.J.; Barros, L.; Ferreira, I.C.F.R. Ultrasound as a rapid and low-cost extraction procedure to obtain anthocyanin-based colorants from Prunus spinosa L. Fruit epicarp: Comparative study with conventional heat-based extraction. Molecules 2019, 24, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daneshfar, A.; Baghlani, M.; Sarabi, R.S.; Sahraei, R.; Abassi, S.; Kaviyan, H.; Khezeli, T. Solubility of citric, malonic, and malic acids in different solvents from 303.2 to 333.2K. Fluid Phase Equilib. 2012, 313, 11–15. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Pereira, E.; Jabeur, I.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Optimization of heat- and ultrasound-assisted extraction of anthocyanins from Hibiscus sabdariffa calyces for natural food colorants. Food Chem. 2019, 275, 309–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iberahim, N.; Sethupathi, S.; Goh, C.L.; Bashir, M.J.K.; Ahmad, W. Optimization of activated palm oil sludge biochar preparation for sulphur dioxide adsorption. J. Environ. Manag. 2019, 248, 109302. [Google Scholar] [CrossRef]
- Vatandas, M.; Koc, A.B.; Koc, C. Ultrasonic velocity measurements in ethanol-water and methanol-water mixtures. Eur. Food Res. Technol. 2007, 225, 525–532. [Google Scholar] [CrossRef]
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Modern extraction and purification techniques for obtaining high purity food-grade bioactive compounds and value-added co-products from citrus wastes. Foods 2019, 8, 523. [Google Scholar] [CrossRef] [Green Version]
- Papoutsis, K.; Golding, J.B.; Vuong, Q.; Pristijono, P.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M. Encapsulation of citrus by-product extracts by spray-drying and freeze-drying using combinations of maltodextrin with soybean protein and ι-carrageenan. Foods 2018, 7, 115. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Tsubaki, S.; Ogawa, K.; Onishi, K.; Azuma, J.I. Isolation of hesperidin from peels of thinned Citrus unshiu fruits by microwave-assisted extraction. Food Chem. 2010, 123, 542–547. [Google Scholar] [CrossRef]
- Assefa, A.D.; Saini, R.K.; Keum, Y.S. Extraction of antioxidants and flavonoids from yuzu (Citrus junos Sieb ex Tanaka) peels: A response surface methodology study. J. Food Meas. Charact. 2017, 11, 364–379. [Google Scholar] [CrossRef]
- Montero-Calderon, A.; Cortes, C.; Zulueta, A.; Frigola, A.; Esteve, M.J. Green solvents and Ultrasound-Assisted Extraction of bioactive orange (Citrus sinensis) peel compounds. Sci. Rep. 2019, 9, 16120. [Google Scholar] [CrossRef]
- Pereira, E.; Encina-Zelada, C.; Barros, L.; Gonzales-Barron, U.; Cadavez, V.; Ferreira, I.C. Chemical and nutritional characterization of Chenopodium quinoa Willd (quinoa) grains: A good alternative to nutritious food. Food Chem. 2019, 280, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Sweis, I.E.; Cressey, B.C. Potential role of the common food additive manufactured citric acid in eliciting significant inflammatory reactions contributing to serious disease states: A series of four case reports. Toxicol. Rep. 2018, 5, 808–812. [Google Scholar] [CrossRef]



| Run | Experimental Design Matrix | Experimental Responses | |||
|---|---|---|---|---|---|
| Time | Ultrasonic Power | Ethanol Proportion | Citric Acid Content (g/100 g Dry Peel) * | ||
| (min) | (W) | (%, v/v) | Orange Peel | Lime Peel | |
| 1 | 11 (−1) | 142 (−1) | 20 (−1) | 4.39 | 1.98 |
| 2 | 36 (+1) | 142 (−1) | 20 (−1) | 5.71 | 2.36 |
| 3 | 11 (−1) | 409 (+1) | 20 (−1) | 5.52 | 2.71 |
| 4 | 36 (+1) | 409 (+1) | 20 (−1) | 4.63 | 2.22 |
| 5 | 11 (−1) | 142 (−1) | 80 (+1) | 1.51 | 0.00 |
| 6 | 36 (+1) | 142 (−1) | 80 (+1) | 2.80 | 0.00 |
| 7 | 11 (−1) | 409 (+1) | 80 (+1) | 2.52 | 0.00 |
| 8 | 36 (+1) | 409 (+1) | 80 (+1) | 2.52 | 0.00 |
| 9 | 2 (−1.68) | 275 (0) | 50 (0) | 2.68 | 2.21 |
| 10 | 45 (+1.68) | 275 (0) | 50 (0) | 3.29 | 2.26 |
| 11 | 24 (0) | 50 (−1.68) | 50 (0) | 2.86 | 0.60 |
| 12 | 24 (0) | 500 (+1.68) | 50 (0) | 3.93 | 0.00 |
| 13 | 24 (0) | 275 (0) | 0 (−1.68) | 6.06 | 2.46 |
| 14 | 24 (0) | 275 (0) | 100 (+1.68) | 1.00 | 0.00 |
| 15 | 24 (0) | 275 (0) | 50 (0) | 3.20 | 0.82 |
| 16 | 24 (0) | 275 (0) | 50 (0) | 3.60 | 0.61 |
| 17 | 24 (0) | 275 (0) | 50 (0) | 3.86 | 0.36 |
| 18 | 24 (0) | 275 (0) | 50 (0) | 3.58 | 0.34 |
| 19 | 24 (0) | 275 (0) | 50 (0) | 3.69 | 0.32 |
| 20 | 24 (0) | 275 (0) | 50 (0) | 3.72 | 0.21 |
| Model Coefficients | Orange Peel | Lime Peel | |
|---|---|---|---|
| Intercept | b0 | 3.55 ± 0.07 | 0.4 ± 0.1 |
| Linear terms | b1 | 0.20 ± 0.08 | 0.05 ± 0.05 * |
| b2 | 0.19 ± 0.08 | ns | |
| b3 | −1.42 ± 0.08 | −0.98 ± 0.08 | |
| Quadratic terms | b11 | ns | 0.61 ± 0.08 |
| b22 | ns | ns | |
| b33 | ns | 0.26 ± 0.08 | |
| Interaction terms | b12 | −0.4 ± 0.1 | ns |
| b13 | ns | ns | |
| b23 | ns | ns | |
| Modeling Statistics | |||
| Model F-value | 69.48 | 48.68 | |
| Lack-of-fit | ns | ns | |
| R2 | 0.9488 | 0.9285 | |
| R2adj | 0.9351 | 0.9094 | |
| Adequate precision | 29.02 | 21.04 | |
| Optimal UAE Conditions | Citric Acid Content | |||
|---|---|---|---|---|
| Time (min) | Ultrasonic Power (W) | Ethanol Proportion (%, v/v) | (g/100 g Dry Peel) | |
| Orange peel | 35.5 | 119.2 | 0.0 | 6.4 ± 0.2 |
| Lime peel | 5.8 | 141.4 | 7.0 | 3.4 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, F.A.; Heleno, S.A.; Pinela, J.; Carocho, M.; Prieto, M.A.; Ferreira, I.C.F.R.; Barros, L. Recovery of Citric Acid from Citrus Peels: Ultrasound-Assisted Extraction Optimized by Response Surface Methodology. Chemosensors 2022, 10, 257. https://doi.org/10.3390/chemosensors10070257
Fernandes FA, Heleno SA, Pinela J, Carocho M, Prieto MA, Ferreira ICFR, Barros L. Recovery of Citric Acid from Citrus Peels: Ultrasound-Assisted Extraction Optimized by Response Surface Methodology. Chemosensors. 2022; 10(7):257. https://doi.org/10.3390/chemosensors10070257
Chicago/Turabian StyleFernandes, Filipa A., Sandrina A. Heleno, José Pinela, Márcio Carocho, Miguel A. Prieto, Isabel C. F. R. Ferreira, and Lillian Barros. 2022. "Recovery of Citric Acid from Citrus Peels: Ultrasound-Assisted Extraction Optimized by Response Surface Methodology" Chemosensors 10, no. 7: 257. https://doi.org/10.3390/chemosensors10070257