Phthalocyanine-Carbon Nanotube Hybrid Materials: Mechanism of Sensor Response to Ammonia from Quantum-Chemical Point of View
Abstract
:1. Introduction
2. Objects and Methods of Investigation
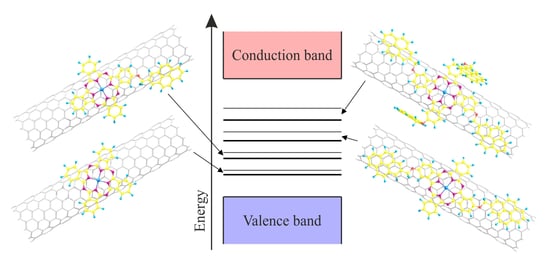
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaporotskova, I.V.; Boroznina, N.P.; Parkhomenko, Y.N.; Kozhitov, L.V. Carbon nanotubes: Sensor properties. A review. Mod. Electron. Mater. 2016, 2, 95–105. [Google Scholar] [CrossRef]
- Norizan, M.N.; Siti Zulaikha, N.D.; Norhana, A.B.; Syakir, M.I.; Norli, A. Carbon nanotubes-based sensor for ammonia gas detection—An overview. Polimery 2021, 66, 175–186. [Google Scholar] [CrossRef]
- Giordano, C.; Filatrella, G.; Sarno, M.; Di Bartolomeo, A. Multi-walled carbon nanotube films for the measurement of the alcoholic concentration. Micro Nano Lett. 2019, 14, 304–308. [Google Scholar] [CrossRef] [Green Version]
- Le, X.V.; Luu, T.L.A.; Nguyen, H.L.; Nguyen, C.T. Synergistic enhancement of ammonia gas-sensing properties at low temperature by compositing carbon nanotubes with tungsten oxide nanobricks. Vacuum 2019, 168, 108861. [Google Scholar] [CrossRef]
- Vu, T.D.; Cong, T.N.; Huu, B.L.; Duc, C.N.; Huu, L.N. Surface-modified carbon nanotubes for enhanced ammonia gas sensitivity at room temperature. J. Nanosci. Nanotechnol. 2019, 19, 7447–7451. [Google Scholar] [CrossRef]
- Norizan, M.N.; Moklis, M.H.; Ngah Demon, S.Z.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef]
- Saxena, S.; Srivastava, A.K.; Srivastava, R.; Kheraj, V. Metal-phthalocyanine functionalized CNTs sensor for chloroform series. Eur. J. Eng. Sci. Technol. 2019, 2, 71–80. [Google Scholar] [CrossRef]
- Ansari, N.; Lone, M.Y.; Shumaila; Ali, J.; Zulfequar, M.; Husain, M.; Islam, S.S.; Husain, S. Trace level toxic ammonia gas sensing of single-walled carbon nanotubes wrapped polyaniline nanofibers. J. Appl. Phys. 2020, 127, 044902. [Google Scholar] [CrossRef]
- Bouanis, F.Z.; Bensifia, M.; Florea, I.; Mahouche-chergui, S.; Carbonnier, B.; Grande, D.; Léonard, C.; Yassar, A.; Pribat, D. Non-covalent functionalization of single walled carbon nanotubes with Fe-/Co-porphyrin and Co-phthalocyanine for field-effect transistor applications. Org. Electron. 2021, 96, 106212. [Google Scholar] [CrossRef]
- Polyakov, M.S.; Basova, T.V. Hybrid materials of zinc (II) tetra-tert-butylphthalocyanine and zinc (II) tetra-tert-butylnaphthalocyanine with single walled carbon nanotubes: Structure and sensing propertie. Macroheterocycles 2017, 10, 31–36. [Google Scholar] [CrossRef]
- Kaya, E.N.; Tuncel, S.; Basova, T.; Banimuslem, H.; Hassan, A.; Gürek, A.G.; Ahsen, V.; Durmuş, M. Effect of pyrene substitution on the formation and sensor properties of phthalocyanine-single walled carbon nanotube hybrids. Sens. Actuators B. Chem. 2014, 199, 277–283. [Google Scholar] [CrossRef]
- Krasnov, P.O.; Ivanova, V.N.; Basova, T.V. Carbon nanotubes functionalized with Zinc (II) phthalocyanines: Effect of the expanded aromatic system and aromatic substituents on the binding energy. Appl. Surf. Sci. 2021, 547, 149172. [Google Scholar] [CrossRef]
- Sharma, A.K.; Mahajan, A.; Bedi, R.K.; Kumar, S.; Debnath, A.K.; Aswal, D.K. Non-covalently anchored multi-walled carbon nanotubes with hexa-decafluorinated zinc p hthalocyanine as ppb level chemiresistive chlorine sensor. Appl. Surf. Sci. 2018, 427, 202–209. [Google Scholar] [CrossRef]
- Saini, R.; Mahajan, A.; Bedi, R.K.; Aswal, D.K.; Debnath, A.K. Room temperature ppb level Cl2 detection and sensing mechanism of highly selective and sensitive phthalocyanine nanowires. Sens. Actuators B. Chem. 2014, 203, 17–24. [Google Scholar] [CrossRef]
- Chia, L.S.; Du, Y.H.; Palale, S.; Lee, P.S. Interaction of copper phthalocyanine with nitrogen dioxide and ammonia investigation using X-ray absorption spectroscopy and chemiresistive gas measurements. ACS Omega 2019, 4, 10388–10395. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wang, X.; Li, X.; Guo, Z.; Zhou, X.; Wu, Y. The effects of amino substituents on the enhanced ammonia sensing performance of PcCo/rGO hybrids. RSC Adv. 2018, 8, 41280–41287. [Google Scholar] [CrossRef] [Green Version]
- Gai, S.; Wang, B.; Wang, X.; Zhang, R.; Miao, S.; Wu, Y. Ultrafast NH3 gas sensor based on phthalocyanine-optimized non-covalent hybrid of carbon nanotubes with pyrrole. Sens. Actuators B. Chem. 2022, 357, 131352. [Google Scholar] [CrossRef]
- Kang, D.; Wang, B.; Wang, X.; Li, Y.; Chen, Z.; He, C.; Wu, Y. Stably dispersed metallophthalocyanine noncovalently bonded to multiwalled carbon nanotubes for ammonia sensing at room temperature. Sens. Actuators B. Chem. 2017, 246, 262–270. [Google Scholar] [CrossRef]
- Prasongkit, J.; Tangsukworakhun, S.; Jaisutti, R.; Osotchan, T. Highly sensitive and selective sensing of acetone and hydrogen sulfide using metal phthalocyanine—carbon nanotube hybrids. Appl. Surf. Sci. 2020, 532, 147314. [Google Scholar] [CrossRef]
- Basiuk, E.V.; Huerta, L.; Basiuk, V.A. Noncovalent bonding of 3d metal (II) phthalocyanines with single-walled carbon nanotubes: A combined DFT and XPS study. Appl. Surf. Sci. 2019, 470, 622–630. [Google Scholar] [CrossRef]
- Mendoza-Domínguez, C.U.; Basiuk, V.A. Adsorption of yttrium bisphthalocyanine on pristine and defect-contaning graphene models: A DFT study. Diam. Relat. Mater. 2022, 126, 109051. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.; Yu, Z.; Zhou, X.; Kang, D.; Wu, Y.; Chen, Z.; He, C.; Zhou, X. The effects of central metals on ammonia sensing of metallophthalocyanines covalently bonded to graphene oxide hybrids. RSC Adv. 2017, 7, 34215–34225. [Google Scholar] [CrossRef] [Green Version]
- Ridhi, R.; Neeru; Gautam, S.; Saini, G.S.S.; Tripathi, S.K.; Rawat, J.S.; Jha, P. Study of the effect of orbital on interaction behaviour of SWCNT-metal phthalocyanines composites with ammonia gas. Sens. Actuators B. Chem. 2021, 337, 129767. [Google Scholar] [CrossRef]
- Dasari, B.S.; Taube, W.R.; Agarwal, P.B.; Rajput, M.; Kumar, A.; Akhtar, J. Room temperature single walled carbon nanotubes (SWCNT) chemiresistive ammonia gas sensor. Sens. Transducers 2015, 190, 24–30. [Google Scholar]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor gas sensors: Materials, technology, design, and application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef]
- Kaya, E.N.; Basova, T.; Polyakov, M.; Durmuş, M.; Hassan, A. Hybrid materials of pyrene substituted phthalocyanines with single-walled carbon nanotubes: Structure and sensing properties. RSC Adv. 2015, 5, 91855–91862. [Google Scholar] [CrossRef]
- Polyakov, M.S.; Basova, T.V.; Göksel, M.; Şenocak, A.; Demirbaş, E.; Durmuş, M.; Kadem, B.; Hassan, A. Effect of covalent and non-covalent linking of zinc(II) phthalocyanine functionalised carbon nanomaterials on the sensor response to ammonia. Synth. Met. 2017, 227, 78–86. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, B.; Wang, X.; Li, Y.; Gai, S.; Wu, Y.; Cheng, X. A high-sensitive room temperature gas sensor based on cobalt phthalocyanines and reduced graphene oxide nanohybrids for the ppb-levels of ammonia detection. RSC Adv. 2019, 9, 37518–37525. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Z.M.; Zhang, J.L.; Wu, F.; He, C.H.; Ren, Z.Y.; Wu, Y.Q. Manipulating polyaniline fibrous networks by doping tetra-β-carboxyphthalocyanine cobalt(II) for remarkably enhanced ammonia sensing. Chem. Mater. 2017, 29, 9509–9517. [Google Scholar] [CrossRef]
- Tai, H.; Duan, Z.; He, Z.; Xian, L.; Xu, J.; Liu, B.; Jiang, Y. Enhanced ammonia response of Ti3C2Tx nanosheets supported by TiO2 nanoparticles at room temperature. Sens. Actuators B. Chem. 2019, 298, 126874. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, C.; Li, P.; Sun, Y. Layer-by-Layer Self-assembly of Co3O4 Nanorod-Decorated MoS2 Nanosheet-Based Nanocomposite toward High-Performance Ammonia Detection. ACS Appl. Mater. Interfaces 2017, 9, 6462–6471. [Google Scholar] [CrossRef] [PubMed]
- Ridhi, R.; Singh, S.; Saini, G.S.S.; Tripathi, S.K. Comparison of interaction mechanisms of copper phthalocyanine and nickel phthalocyanine thin films with chemical vapours. J. Phys. Chem. Solids 2018, 115, 119–126. [Google Scholar] [CrossRef]
- Liang, X.; Chen, Z.; Wu, H.; Guo, L.; He, C.; Wang, B.; Wu, Y. Enhanced NH3-sensing behavior of 2,9,16,23-tetrakis(2,2,3,3-tetrafluoropropoxy) metal(II) phthalocyanine/multi-walled carbon nanotube hybrids: An investigation of the effects of central metals. Carbon 2014, 80, 268–278. [Google Scholar] [CrossRef]
- Cao, Z.; Chen, Q.; Lu, Y.; Liu, H.; Hu, Y. Density functional theory study on the interaction between metalloporphyrins and NH3. Int. J. Quantum Chem. 2012, 113, 1137–1146. [Google Scholar] [CrossRef]
- Baggio, A.R.; Machado, D.F.S.; Carvalho-Silva, V.H.; Paterno, L.G.; de Oliveira, H.C.B. Rovibrational spectroscopic constants of the interaction between ammonia and metallo-phthalocyanines: A theoretical protocol for ammonia sensor design. Phys. Chem. Chem. Phys. 2017, 19, 10843–10853. [Google Scholar] [CrossRef]
- Rana, M.K.; Sinha, M.; Panda, S. Gas sensing behavior of metal-phthalocyanines: Effects of electronic structure on sensitivity. Chem. Phys. 2018, 513, 23–24. [Google Scholar] [CrossRef]
- Kaya, E.N.; Şenocak, A.; Klyamer, D.D.; Demirbaş, E.; Basova, T.V.; Durmuş, M. Ammonia sensing performance of thin films of cobalt(II) phthalocyanine bearing fluorinated substituents. J. Mater. Sci. Mater. Electron. 2019, 30, 7543–7551. [Google Scholar] [CrossRef]
- Ivanova, V.; Klyamer, D.; Krasnov, P.; Kaya, E.N.; Kulu, İ.; Kostakoglu, S.T.; Durmuş, M.; Basova, T. Hybrid materials based on pyrene-substituted metallo phthalocyanines as sensing layers for ammonia detection: Effect of the number of pyrene substituents. Sens. Actuators B. Chem. 2022, 375, 132843. [Google Scholar] [CrossRef]
- Elstner, M.; Porezag, D.; Jungnickel, G.; Elsner, J.; Haugk, M.; Frauenheim, T.; Suhai, S.; Seifert, G. Self-consistent-charge density-functional tight-binding method for simulations of complex materials properties. Phys. Rev. B 1998, 58, 7260–7268. [Google Scholar] [CrossRef]
- Hourahine, B.; Aradi, B.; Blum, V.; Bonafé, F.; Buccheri, A.; Camacho, C.; Cevallos, C.; Deshaye, M.Y.; Dumitric, T.; Dominguez, A.; et al. DFTB+, a software package for efficient approximate density functional theory based atomistic simulations. J. Chem. Phys. 2020, 152, 124101. [Google Scholar] [CrossRef]
- Lu, X.; Gaus, M.; Elstner, M.; Cui, Q. Parametrization of DFTB3/3OB for magnesium and zinc for chemical and biological applications. J. Phys. Chem. B 2015, 119, 1062–1082. [Google Scholar] [CrossRef] [PubMed]
- Gaus, M.; Goez, A.; Elstner, M. Parametrization and benchmark of DFTB3 for organic molecules. J. Chem. Theory Comput. 2013, 9, 338–354. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasnov, P.O.; Basova, T.V.; Hassan, A. Interaction of metal phthalocyanines with carbon zigzag and armchair nanotubes with different diameters. Appl. Surf. Sci. 2018, 457, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillonin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Density functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2017, 8, e1327. [Google Scholar] [CrossRef]
- Baerends, E.J.; Ellis, D.E.; Ros, P. Self-consistent molecular Hartree-Fock-Slater calculations I. The computational procedure. J. Chem. Phys. 1973, 2, 41–51. [Google Scholar] [CrossRef]
- Dunlap, B.I.; Connolly, J.W.D.; Sabin, J.R. On some approximations in applications of Xα theory. J. Chem. Phys. 1979, 71, 3396–3402. [Google Scholar] [CrossRef]
- Van Alsenoy, C. Ab initio calculations on large molecules: The multiplicative integral approximation. J. Comp. Chem. 1988, 9, 620–626. [Google Scholar] [CrossRef]
- Kendall, R.A.; Früchtl, H.A. The impact of the resolution of the identity approximate integral method on modern ab initio algorithm development. Theor. Chem. Acc. 1997, 97, 158–163. [Google Scholar] [CrossRef]
- Eichkorn, K.; Treutler, O.; Öhm, H. Auxiliary basis sets to approximate Coulomb potentials. Chem. Phys. Lett. 1995, 240, 283–290. [Google Scholar] [CrossRef]
- Eichkorn, K.; Weigend, F.; Treutler, O. Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials. Theor. Chem. Acc. 1997, 97, 119–124. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Essén, H. The characterization of atomic interactions. J. Chem. Phys. 1984, 80, 1943–1960. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Todd, A.; Keith, T.K. AIMAll, version 19.10.12; Gristmill Software: Overland Park, KS, USA, 2019; Available online: aim.tkgristmill.com (accessed on 24 October 2022).
- Bushmarinov, I.S.; Lyssenko, K.A.; Antipin, M.Y. Atomic energy in the ’Atoms in Molecules’ theory and its use for solving chemical problems. Russ. Chem. Rev. 2009, 78, 283–302. [Google Scholar] [CrossRef]




| x | Eg1, eV | Eg2, eV | ΔE, eV | n1/n2 |
|---|---|---|---|---|
| 0 | 0.774 | 0.774 | 0.005 | 1.10 |
| 1 | 0.774 | 0.773 | 0.013 | 1.29 |
| 2 | 0.775 | 0.774 | 0.019 | 1.45 |
| 4 | 0.772 | 0.772 | 0.038 | 2.10 |
| x | Position Number of the NH3 Molecule (in Accordance with Figure 2) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| 0 | 0.141 | 0.141 | 0.141 | 0.141 |
| 1 | 0.133 | 0.127 | 0.141 | 0.215 |
| 2 | 0.139 | 0.149 | 0.145 | 0.204 |
| 4 | 0.156 | 0.156 | 0.156 | 0.156 |
| BCP | Atoms | ρ(r), e/Å3 | ∇2ρ(r), e/Å5 | q, e |
|---|---|---|---|---|
| 1 | N1-H1 | 0.146 | 1.602 | 0.148 |
| 2 | N2-H2 | 0.117 | 1.162 | - |
| 3 | N1-H3 | 0.096 | 1.050 | 0.051 |
| 4 | N3-H4 | 0.140 | 1.543 | 0.106 |
| 5 | N4-H5 | 0.129 | 1.270 | - |
| 6 | N3-H6 | 0.098 | 1.069 | 0.046 |
| 7 | N5-H7 | 0.137 | 1.527 | 0.107 |
| 8 | N6-H8 | 0.125 | 1.238 | - |
| 9 | N5-H9 | 0.100 | 1.092 | 0.047 |
| 10 | N7-H10 | 0.138 | 1.530 | 0.107 |
| 11 | N8-H11 | 0.126 | 1.250 | - |
| 12 | N7-H12 | 0.099 | 1.082 | 0.048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasnov, P.; Ivanova, V.; Klyamer, D.; Fedorov, A.; Basova, T. Phthalocyanine-Carbon Nanotube Hybrid Materials: Mechanism of Sensor Response to Ammonia from Quantum-Chemical Point of View. Chemosensors 2022, 10, 479. https://doi.org/10.3390/chemosensors10110479
Krasnov P, Ivanova V, Klyamer D, Fedorov A, Basova T. Phthalocyanine-Carbon Nanotube Hybrid Materials: Mechanism of Sensor Response to Ammonia from Quantum-Chemical Point of View. Chemosensors. 2022; 10(11):479. https://doi.org/10.3390/chemosensors10110479
Chicago/Turabian StyleKrasnov, Pavel, Victoria Ivanova, Darya Klyamer, Aleksandr Fedorov, and Tamara Basova. 2022. "Phthalocyanine-Carbon Nanotube Hybrid Materials: Mechanism of Sensor Response to Ammonia from Quantum-Chemical Point of View" Chemosensors 10, no. 11: 479. https://doi.org/10.3390/chemosensors10110479