Improving the Standard of Care for All—A Practical Guide to Developing a Center of Excellence
Abstract
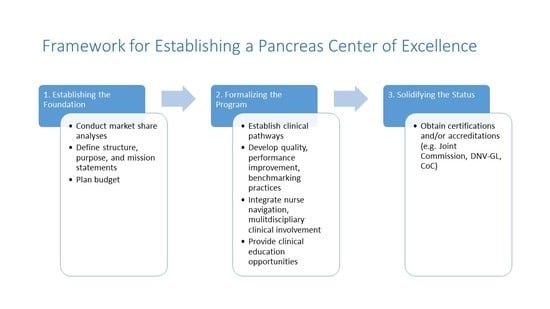
:1. Introduction
- •
- Establishing the foundation (i.e., leadership structure and purpose; financial considerations);
- •
- Formalizing the program (i.e., clinical education and competency training; nurse navigation and multidisciplinary involvement; objective measures of clinical excellence; quality and performance improvement initiatives);
- •
- Solidifying the CoE status (i.e., certification/accreditation by external institutions; marketing and outreach).
2. Establishing the Foundation
2.1. Leadership Structure and Purpose
2.2. Timeline of Pancreas CoE Development
- •
- Provide the highest standard of care, services, and support to each patient;
- •
- Communicate process improvements and data to key stake holders in the pancreas domain;
- •
- Analyze barriers and data to create better clinical pathways and care maps;
- •
- Identify best practice guidelines and use them in our pancreas population;
- •
- Identify quality and utilization metrics used to analyze physician practices.
2.3. Determining CoE’s Market Share
2.4. Technology and Cost Considerations
3. Formalizing the Program
3.1. Education/Competency/Training
3.2. Multidisciplinary Involvement
3.3. Clinical Information Systems
3.4. Value-Based Healthcare
3.5. Quality/Performance Improvement Outcomes
4. Solidifying the CoE Status
4.1. Certifications and Designations
4.2. Marketing a CoE
- •
- The PLC is a beneficial tool that helps marketers manage the stages of a product’s acceptance and success in the marketplace. The PLC begins with the product’s introduction and continues through its growth in market share, maturity, and possible decline in market share [60]. Where the CoE lands in the program life cycle (e.g., introductory, growth, maturity, or decline) must be evaluated to help determine appropriate messaging.
- •
- Legal and ethical issues: Prior to embarking on CoE marketing, it is important to consider the legal and ethical ramifications of medical marketing. Consider the following questions with an organization’s legal and marketing teams:
- ○
- Are all quality claims backed by evidence-based criteria? Objective claims regarding experience, competence, and the quality of physicians and the services they provide may only be made if they are factually supportable [61]. In addition, be mindful that marketing materials, including websites, should be reviewed and updated regularly (at least annually, or as changes occur) to ensure that claims continue to be accurate.
- ○
- What are the organization’s policies regarding advertising individual providers?
- ○
- Are there disclaimers that must be used in marketing materials?
- ○
- Do brand guidelines dictate the identity of services?
- •
- Provider expertise: Several factors lend to the reputation of an organizations’ providers, and subsequently the program. One factor is being a teaching facility, such as the Intuitive Surgical Training Epicenters, or having surgeons who are board-certified and fellowship-trained. Another factor is identifying physician leaders, champions, and subject matter experts among specialists to determine those most able and willing to help develop communications materials and participate in marketing efforts.
- •
- Defining the market and audience: Identify the target audience before planning a promotional mix. Both providers (e.g., physicians, physician assistants, and nurse practitioners) and direct consumers comprise the target audience for services. Messaging should be crafted to meet the needs and level of understanding of the target audience.
- •
- Setting marketing goals: Establish baselines for marketing metrics and develop SMART goals for each. Goals might include increasing procedure volume, increasing referrals, expanding referral base, increase market share, and achieving return on investment from marketing expenditures.
- •
- Budgeting and tracking results: Before marketing the CoE, know the organization’s budgeting cycle. The budget will dictate the promotional mix. A marketing consultant can provide cost estimates for the tools and tactics they recommend. Results should be tracked against the original baseline SMART goals and metrics established at the onset of planning efforts.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nassour, I.; Choti, M.A. Pancreatic Operations. JAMA 2016, 316, 1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zovak, M.; Mužina Mišić, D.; Glavčić, G. Pancreatic surgery: Evolution and current tailored approach. Hepatobiliary Surg. Nutr. 2014, 3, 247–258. [Google Scholar] [CrossRef]
- Nimptsch, U.; Krautz, C.; Weber, G.F.; Mansky, T.; Grutzmann, R. Nationwide In-hospital Mortality Following Pancreatic Surgery in Germany is Higher than Anticipated. Ann. Surg. 2016, 264, 1082–1090. [Google Scholar] [CrossRef]
- Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP); Agency for Healthcare Research and Quality: Rockville, MD, USA, 2019.
- Tan-Tam, C.; Segedi, M.; Chung, S. Whipple procedure: Patient selection and special considerations. Open Access Surg. 2016, 2016, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Gagner, M.; Pomp, A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg. Endosc. 1994, 8, 408–410. [Google Scholar] [CrossRef]
- Giulianotti, P.C.; Coratti, A.; Angelini, M.; Sbrana, F.; Cecconi, S.; Balestracci, T.; Caravaglios, G. Robotics in general surgery: Personal experience in a large community hospital. Arch. Surg. 2003, 138, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Thornblade, L.W.; Shi, X.; Ruiz, A.; Flum, D.R.; Park, J.O. Comparative Effectiveness of Minimally Invasive Surgery and Conventional Approaches for Major or Challenging Hepatectomy. J. Am. Coll. Surg. 2017, 224, 851–861. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.T.; Gamblin, T.C.; Geller, D.A. World review of laparoscopic liver resection-2804 patients. Ann. Urgery 2009, 250, 831–841. [Google Scholar]
- Schiffman, S.C.; Kim, K.H.; Tsung, A.; Marsh, J.W.; Geller, D.A. Laparoscopic versus open liver resection for metastatic colorectal cancer: A metaanalysis of 610 patients. Surgery 2015, 157, 211–222. [Google Scholar] [CrossRef]
- Sham, J.G.; Richards, M.K.; Seo, Y.D.; Pillarisetty, V.G.; Yeung, R.S.; Park, J.O. Efficacy and cost of robotic hepatectomy: Is the robot cost-prohibitive? J. Robot. Surg. 2016, 10, 307–313. [Google Scholar] [CrossRef]
- Bagante, F.; Spolverato, G.; Strasberg, S.M.; Gani, F.; Thompson, V.; Hall, B.L.; Bentrem, D.J.; Pitt, H.A.; Pawlik, T.M. Minimally Invasive vs. Open Hepatectomy: A Comparative Analysis of the National Surgical Quality Improvement Program Database. J. Gastrointest. Surg. 2016, 20, 1608–1617. [Google Scholar] [CrossRef]
- Rao, A.; Rao, G.; Ahmed, I. Laparoscopic vs. open liver resection for maignant liver disease. A systematic review. Surgeon 2012, 10, 194–201. [Google Scholar] [CrossRef]
- Yadav, D.; Lowenfels, A.B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013, 144, 1252–1261. [Google Scholar] [CrossRef] [Green Version]
- Munigala, S.; Yadav, D. Case-fatality from acute pancreatitis is decreasing but its population mortality shows little change. Pancreatology 2016, 16, 542–550. [Google Scholar] [CrossRef] [Green Version]
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Lund, J.L.; Dellon, E.S.; Williams, J.L.; Jensen, E.T.; Shaheen, N.J.; Barritt, A.S.; Lieber, S.R.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology 2019, 156, 254–272.e211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peery, A.F.; Dellon, E.S.; Lund, J.; Crockett, S.D.; McGowan, C.E.; Bulsiewicz, W.J.; Gangarosa, L.M.; Thiny, M.T.; Stizenberg, K.; Morgan, D.R.; et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012, 143, 1179–1187.e1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.S.; Chung, M.J.; Park, J.Y.; Bang, S.; Park, S.W.; Song, S.Y.; Chung, J.B. Urgent endoscopic retrograde cholangiopancreatography is not superior to early ERCP in acute biliary pancreatitis with biliary obstruction without cholangitis. PLoS ONE 2018, 13, e0190835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenner, S.; Baillie, J.; DeWitt, J.; Vege, S.S. American College of Gastroenterology guideline: Management of acute pancreatitis. Am. J. Gastroenterol. 2013, 108, 1400–1416. [Google Scholar] [CrossRef]
- Vivian, E.; Cler, L.; Conwell, D.; Cote, G.A.; Dickerman, R.; Freeman, M.; Gardner, T.B.; Hawes, R.H.; Kedia, P.; Krishnamoorthi, R.; et al. Acute Pancreatitis Task Force on Quality: Development of Quality Indicators for Acute Pancreatitis Management. Am. J. Gastroenterol. 2019, 114, 1322–1342. [Google Scholar] [CrossRef]
- Jovanovic, I.; Mönkemüller, K. Quality in endoscopy training-the endoscopic retrograde cholangiopancreatography case. Ann. Transl. Med. 2018, 6, 264. [Google Scholar] [CrossRef]
- Cho, C.M.; Dewitt, J.; Al-Haddad, M. Echo-endoscopy: New therapeutic frontiers. Minerva Gastroenterol. Dietol. 2011, 57, 139–158. [Google Scholar] [PubMed]
- Lieberman, M.D.; Kilburn, H.; Lindsey, M.; Brennan, M.F. Relation of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann. Surg. 1995, 222, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Orr, R.K. Outcomes in pancreatic cancer surgery. Surg. Clin. N. Am. 2010, 90, 219–234. [Google Scholar] [CrossRef]
- Adam, M.A.; Thomas, S.; Youngwirth, L.; Pappas, T.; Roman, S.A.; Sosa, J.A. Defining a Hospital Volume Threshold for Minimally Invasive Pancreaticoduodenectomy in the United States. JAMA Surg. 2017, 152, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, V.E.P.P.; Bosscha, K.; van der Schelling, G.; Brenninkmeijer, S.; Coebergh, J.W.W.; de Hingh, I.H.J.T. Improving outcome for patients with pancreatic cancer through centralization. BJS 2011, 98, 1455–1462. [Google Scholar] [CrossRef]
- Coté, G.A.; Imler, T.D.; Xu, H.; Teal, E.; French, D.D.; Imperiale, T.F.; Rosenman, M.B.; Wilson, J.; Hui, S.L.; Sherman, S. Lower provider volume is associated with higher failure rates for endoscopic retrograde cholangiopancreatography. Med. Care 2013, 51, 1040–1047. [Google Scholar] [CrossRef] [Green Version]
- Keswani, R.N.; Qumseya, B.J.; O’Dwyer, L.C.; Wani, S. Association Between Endoscopist and Center Endoscopic Retrograde Cholangiopancreatography Volume With Procedure Success and Adverse Outcomes: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2017, 15, 1866–1875.e1863. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.J.; Barakat, M.T.; Girotra, M.; Lee, J.S.; Banerjee, S. Unplanned Hospital Encounters After Endoscopic Retrograde Cholangiopancreatography in 3 Large North American States. Gastroenterology 2019, 156, 119–129.e113. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, C.M.; Heo, J.; Jung, M.K.; Kim, T.N.; Kim, K.H.; Kim, H.; Cho, K.B.; Kim, H.G.; Han, J.; et al. Impact of Hospital Volume and the Experience of Endoscopist on Adverse Events Related to Endoscopic Retrograde Cholangiopancreatography: A Prospective Observational Study. Gut Liver 2020, 14, 257–264. [Google Scholar] [CrossRef] [Green Version]
- D’Angelica, M.I.; Chapman, W.C. HPB Surgery: The Specialty is Here to Stay, but the Training is in Evolution. Ann. Surg. Oncol. 2016, 23, 2123–2125. [Google Scholar] [CrossRef] [Green Version]
- Why Is a ‘Center of Excellence’ Different from an Institute? Available online: https://www.advisory.com/research/market-innovation-center/the-growth-channel/09/what-is-the-difference-between-a-center-of-excellence-and-an-institute (accessed on 15 May 2019).
- Elrod, J.K.; Fortenberry, J.L., Jr. Centers of excellence in healthcare institutions: What they are and how to assemble them. BMC Health Serv. Res. 2017, 17, 425. [Google Scholar] [CrossRef] [PubMed]
- Bata, S.A.; Richardson, T. Value of Investment as a Key Driver for Prioritization and Implementation of Healthcare Software. Perspect. Health Inf. Manag. 2018, 15, 4. [Google Scholar]
- Perez, R.E.; Schwaitzberg, S.D. Robotic surgery: Finding value in 2019 and beyond. Ann. Laparosc. Endosc. Surg. 2019, 4, 51. [Google Scholar] [CrossRef]
- Leddy, L.S.; Lendvay, T.S.; Satava, R.M. Robotic surgery: Applications and cost effectiveness. Open Access Surg. 2010, 3, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Mejia, A.; Shah, J.; Vivian, E.; Acharya, P. Analysis of 102 Fully Robotic Pancreaticoduodenectomies: Clinical and Financial Outcomes. Pancreas 2020, 49, 668–674. [Google Scholar] [CrossRef]
- SpyGlass™ DS—Direct Visualization System. Available online: https://www.bostonscientific.com/content/gwc/en-US/products/direct-visualization-systems/spyglass-ds-direct-visualization-system.html (accessed on 20 July 2020).
- Wong, J.C.; Tang, R.S.; Teoh, A.Y.; Sung, J.J.; Lau, J.Y. Efficacy and safety of novel digital single-operator peroral cholangioscopy-guided laser lithotripsy for complicated biliary stones. Endosc. Int. Open 2017, 5, E54–E58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brewer Gutierrez, O.I.; Bekkali, N.L.H.; Raijman, I.; Sturgess, R.; Sejpal, D.V.; Aridi, H.D.; Sherman, S.; Shah, R.J.; Kwon, R.S.; Buxbaum, J.L.; et al. Efficacy and Safety of Digital Single-Operator Cholangioscopy for Difficult Biliary Stones. Clin. Gastroenterol. Hepatol. 2018, 16, 918–926.e911. [Google Scholar] [CrossRef]
- Hendren, S.; Fiscella, K. Patient Navigation Improves the Care Experience for Patients With Newly Diagnosed Cancer. J. Clin. Oncol. 2013, 32, 3–4. [Google Scholar] [CrossRef]
- Harding, M. Effect of nurse navigation on patient care satisfaction and distress associated with breast biopsy. Clin. J. Oncol. Nurs. 2015, 19, E15–E20. [Google Scholar] [CrossRef]
- Campbell, C.; Craig, J.; Eggert, J.; Bailey-Dorton, C. Implementing and measuring the impact of patient navigation at a comprehensive community cancer center. Oncol. Nurs. Forum 2010, 37, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Wells, K.J.; Battaglia, T.A.; Dudley, D.J.; Garcia, R.; Greene, A.; Calhoun, E.; Mandelblatt, J.S.; Paskett, E.D.; Raich, P.C.; Patient Navigation Research, P. Patient navigation: State of the art or is it science? Cancer 2008, 113, 1999–2010. [Google Scholar] [CrossRef] [Green Version]
- Schafer, J.M.; Swisher, J. Clinical Intelligence: Cancer Care Coordination with Nurse Navigators; SG-2®, LLC: Skokie, IL, USA, 2006. [Google Scholar]
- Gillis, C.; Wischmeyer, P.E. Pre-operative nutrition and the elective surgical patient: Why, how and what? Anaesthesia 2019, 74 (Suppl. 1), 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weggelaar-Jansen, A.; Broekharst, D.S.E.; de Bruijne, M. Developing a hospital-wide quality and safety dashboard: A qualitative research study. BMJ Qual. Saf. 2018, 27, 1000–1007. [Google Scholar] [CrossRef] [Green Version]
- Dowding, D.; Randell, R.; Gardner, P.; Fitzpatrick, G.; Dykes, P.; Favela, J.; Hamer, S.; Whitewood-Moores, Z.; Hardiker, N.; Borycki, E.; et al. Dashboards for improving patient care: Review of the literature. Int. J. Med. Inform. 2015, 84, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Ivers, N.M.; Barrett, J. Using report cards and dashboards to drive quality improvement: Lessons learnt and lessons still to learn. BMJ Qual. Saf. 2018, 27, 417–420. [Google Scholar] [CrossRef] [Green Version]
- Chee, T.T.; Ryan, A.M.; Wasfy, J.H.; Borden, W.B. Current State of Value-Based Purchasing Programs. Circulation 2016, 133, 2197–2205. [Google Scholar] [CrossRef] [Green Version]
- Porter, M.E. Redefining Health Care: Creating Value-Based Competition on Results; National Association of Chain Drug Stores Annual Meeting; Harvard Business Review Press: Brighton, MA, USA, 2006. [Google Scholar]
- Wong, J.; Chan, I. Engaging Physicians Using Value Management Tools—NUHS Experience. In Proceedings of the Institute for Healthcare Improvement National Forum, Orlando, FL, USA, 9–12 December 2018. [Google Scholar]
- Lee, V.S.; Kawamoto, K.; Hess, R.; Park, C.; Young, J.; Hunter, C.; Johnson, S.; Gulbransen, S.; Pelt, C.E.; Horton, D.J.; et al. Implementation of a Value-Driven Outcomes Program to Identify High Variability in Clinical Costs and Outcomes and Association With Reduced Cost and Improved Quality. JAMA 2016, 316, 1061–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, J.; Nwogu, C.; Vivian, E.; John, E.S.; Kedia, P.; Sellers, B.; Cler, L.; Acharya, P.; Tarnasky, P. The Value of Managing Acute Pancreatitis With Standardized Order Sets to Achieve “Perfect Care”. Pancreas 2021, 50, 293–299. [Google Scholar] [CrossRef]
- Steele, S.; Branstetter, H.; Shah, J.; Acharya, P.; Avila, N.; Khan, H.; Reddy, R.; Muller, M.; Kedia, P.; Tarnasky, P. Initial Imaging in Patients with Acute Pancreatitis: Impact of Quality Improvement. In Proceedings of the World Congress of Gastroenterology at ACG, Orlando, FL, USA, 13–18 October 2017. [Google Scholar]
- Benefits of Joint Commission Certification. Available online: https://www.jointcommission.org/benefits_of_joint_commission_certification/ (accessed on 17 June 2021).
- Facts about The Joint Commission. Available online: https://www.jointcommission.org/facts_about_the_joint_commission/ (accessed on 15 June 2020).
- DNV GL Healthcare Program Certifications. Available online: https://www.dnvgl.us/assurance/healthcare/certifications.html (accessed on 23 August 2019).
- Methodist Dallas Medical Center Named General Surgery Epicenter Focusing on Liver and Pancreas Robotic-Assisted Surgery. Available online: https://www.methodisthealthsystem.org/news-center/2015/february/methodist-dallas-medical-center-named-general-su/ (accessed on 23 August 2019).
- Managing New Products: The Product Life Cycle. Available online: https://open.lib.umn.edu/principlesmarketing/chapter/7-2-managing-new-products-the-product-life-cycle/ (accessed on 8 May 2019).
- Ethics: Advertising & Publicity. Available online: https://www.ama-assn.org/delivering-care/ethics/advertising-publicity (accessed on 8 May 2019).





| Perfect Care Index Metric | Goal |
|---|---|
| Clinical Quality and Safety | |
| In-hospital mortality | No |
| 30-day readmission | No |
| Length of stay | ≤Expected based on risk-adjusted data |
| Final severity of pancreatitis | Mild or moderate severity |
| Process of Care | |
| Computed tomography scan ordered in the Emergency Department | No |
| Lactated ringer’s administered in the Emergency Department | Yes |
| ERCP † performed within 24 h for patients with cholangitis | Yes |
| BUN ‡ and HCT § | Decrease from day 0 to day 1 |
| Total parenteral nutrition usage | No |
| Oral nutrition | Within 72 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vivian, E.; Brooks, M.R.; Longoria, R.; Lundberg, L.; Mallow, J.; Shah, J.; Vo, A.; Mejia, A.; Tarnasky, P.; Puri, V. Improving the Standard of Care for All—A Practical Guide to Developing a Center of Excellence. Healthcare 2021, 9, 777. https://doi.org/10.3390/healthcare9060777
Vivian E, Brooks MR, Longoria R, Lundberg L, Mallow J, Shah J, Vo A, Mejia A, Tarnasky P, Puri V. Improving the Standard of Care for All—A Practical Guide to Developing a Center of Excellence. Healthcare. 2021; 9(6):777. https://doi.org/10.3390/healthcare9060777
Chicago/Turabian StyleVivian, Elaina, Mary Rachel Brooks, Raquel Longoria, Laurie Lundberg, Jenifer Mallow, Jimmy Shah, Allison Vo, Alejandro Mejia, Paul Tarnasky, and Vichin Puri. 2021. "Improving the Standard of Care for All—A Practical Guide to Developing a Center of Excellence" Healthcare 9, no. 6: 777. https://doi.org/10.3390/healthcare9060777