1. Introduction
The resorptive potential of orthodontic treatment is acknowledged as a common, inevitable, and unpredictable side effect in both the periodontium and tooth [
1,
2]. The orthodontic movement is initiated by circulatory disturbances at the periodontal ligament (PDL) level due to variations in the physiological maximum hydrostatic pressure (MHP) [
2,
3].
The effects of the orthodontic forces in PDL are various levels of ischemia (the higher the force, the stronger the level of ischemia seemed to be), stimulating the initiation of movements, ischemic circulatory disturbances in dental pulp, neuro-vascular bundles (NVB), and further periodontal loss (strictly correlated with the levels of the already present bone loss) [
3,
4,
5].
In the tooth, the effects of the orthodontic force are related mainly to the appearance of resorptive areas/lacunae both on the external and internal surface of the root, root canals, and pulp chamber (the higher the applied force, the faster and more extensive the resorptive areas developed) [
6,
7,
8,
9].
There are close correlations and relationships between the amount of the applied orthodontic force and the maximum hydrostatic pressure (MHP) in the PDL and dental pulp–NVB tissues. The MHP was reported to be 12.8–16 KPa (about 80% of the systolic pressure), and it is advised that this not be exceeded (to avoid ischemic loss), while it is recommended that the minimum hydrostatic pressure of 4.7 KPa be surpassed in order to trigger the movement [
3,
4,
5,
6,
7,
10,
11,
12]. There are numerous reports regarding the optimal applied force of about 1 N (light forces of 0.5–1 N/approx. 50–100 gf), triggering the orthodontic movements but without significant ischemic and resorptive risks [
2,
4,
5,
6,
8,
10,
11,
13,
14]. Opposingly, other studies adopt as optimal strengths amounts of force much higher without reporting any major resorptive/ischemic/necrotic risks 0.28–3.31 N/approx. 28–331 gf [
15,
16,
17], considering that the light forces do not effectively trigger the alveolar bone remodeling processes [
6].
Nevertheless, it is acknowledged that the high amounts of applied force produce localized points/areas of high pressure (where the stresses produced by the applied force are high) over the external and internal surface of the tooth, with the results of the internal and external orthodontic resorptive processes [
6,
7,
18] continuing another 4 weeks after the force action has stopped [
1].
The prevalence of the resorptive process (affecting both the root and crown [
2,
6,
14]) due to orthodontic causes was reported to be variable, with reports between 0.02–2.3% (external cervical) and 1–5% (external radicular) [
2], and 1–51.5% (internal radicular) [
2], up to 20–100% [
1,
7,
14]. Usually, the resorptions are unpredictive isolated localized areas/lacunae of various depths and surface extents, with no clinical symptomatology appearing after two–four weeks after applying the orthodontic force [
19,
20]. However, symptoms and signs of acute/chronic pulpitis could be present if the ischemic disturbances are important [
2]. The mechanism is not completely understood and studied; it seems that the source is the ischemic disturbances affecting the MHP, triggering resorptive processes until the pressure source is removed [
2,
14]. There are little data available regarding the internal root resorption and no finite elements studies (FEA) were found regarding this issue [
2].
The internal root resorption seems to be initiated along the root canal with the progressive destruction of the dentine (damage to the odontoblasts and unmineralized pre-dentine) due to orthodontic treatment pressures and localized inflammation of the dental pulp tissue of ischemic orthodontic origin [
2]. The process consists of two phases, the transient phase with a self-limiting resorption, and the progressive phase with ischemic necrosis triggering bacterial resorptive activity [
2]. There are also two types of resorptions: inflammatory internal resorption (i.e., loss of intra-radicular dentine without any deposition in the resorptive lacunae), and replacement internal root resorption (i.e., loss of intra-radicular dentine replaced by metaplastic bone/cementum-like hard tissues) [
2].
The clinical treatment of the internal root resorption depends on the results of the extent of destruction and whether there are present signs of pulp sufferance demanding endodontic root canal treatment and/or regenerative endodontic procedures. If the perforations are present, the endodontic regenerative procedures include calcium hydroxide and/or antibiotic paste for a variable period of 0.5–3 months followed by MTA filling [
2,
9].
The finite elements analysis (FEA) is a well-known study method allowing individual analysis of each component of a structure, especially useful in extremely small structures that cannot be otherwise studied [
4,
5,
8,
9,
10,
11,
12,
21].
The in vivo dental tissues (tooth and surrounding periodontium in particular) are particularly suited for the use of FEA due to their small anatomical dimensions and complex anatomy. FEA is an exact mathematical method, widely used in the engineering field, providing accurate results if the employment conditions and the input data are correct [
4,
5,
8,
9,
10,
11,
12,
21,
22].
In dentistry, FEA studies have often reported debatable results, sometimes contradicting the available clinical data, making this useful study method the object of mistrust due to the misunderstanding of the yielding materials theory (which relies on the failure criteria design). Nonetheless, there are reports that if adequate material-based failure criteria and anatomical correct input data are used, then the results will be accurate and correlate with the in vivo clinical data [
4,
5,
8,
10,
11,
12,
22].
When the dental tissues are FEA-studied, their micro-architecture and physical properties must be acknowledged, as well as their biomechanical behaviors [
3,
13,
22,
23,
24,
25]. When subjected to an amount of force, the tooth components and surrounding periodontium display a remarkable ability of absorption–dissipation, such that only a reduced amount of stress directly affects the surrounding periodontium and dental pulp–NVB [
3,
4,
5,
8,
10,
11,
12,
23,
24,
25,
26,
27]. This biomechanical ability of absorption–dissipation corresponds to ductile materials; otherwise, the dental tissues being reported resemble ductile materials (but with a certain brittle flow mode) [
3,
4,
5,
8,
10,
11,
12,
23,
24,
25,
26,
27].
The debatable reports of the FEA dental studies are due more to the misemployment of the failure criteria and less because of the input data [
3,
8,
10,
11,
21]. The failure criteria are material-based designed (each material type poses a certain biomechanical behavior under stress—the yielding theory) [
3,
8,
10,
11,
21]. The main difference is due to their different deformations under stress, the ductile (e.g., steel, rubber) suffers from elastic/plastic deformation with variable degrees of reversibility before fracture/destruction, while brittle materials (e.g., stone, glass) suffer directly from fracture/destruction [
3,
8,
10,
11,
21]. All dental tissues (dentine, cementum, dental pulp, NVB, and PDL) are considered to resemble ductile, except for the enamel, which is considered to resemble brittle [
4,
5,
8,
10,
11,
12,
23,
24,
25,
26,
27]. Nevertheless, the enamel is only a small % of the tooth structure; thus, the tooth biomechanical behavior resembles that of ductile [
4,
5,
8,
10,
11,
12,
23,
24,
25,
26,
27]. Despite multiple reports and clinical evidence [
4,
5,
8,
10,
11,
12,
23,
24,
25,
26,
27] regarding the ductility resemblance of dentine, there are still studies [
22] that consider dentine to resemble a brittle solid, without further discussion of the issues, suggesting that both maximum principal and von Mises criteria should be used [
22]. Moreover, other studies actively employed hydrostatic stresses and reported them to be the only adequate criteria, reporting different results from one study to another and for the same issues [
15,
16,
17,
28,
29].
There are many FEA studies of PDL, while for the external root resorption, only a few were available, and none for the external and internal root resorption in periodontal breakdown. No studies related to the resorption process (neither external nor internal) were found for the periodontal breakdown despite various bone levels being found in orthodontic patients. Moreover, no information about the amount of safely applied orthodontic force to avoid orthodontic resorption or absorption–dissipation ability was found. These studies used various failure criteria (von Mises [
9,
21,
30,
31], maximum principal S1 tensile stress/minimum principal S3 compressive stress [
21,
30,
31,
32,
33], and hydrostatic pressure [
6,
7,
15,
16,
17,
28,
29,
30,
31,
34]), without any correlation with the type of analyzed material, MHP, ischemic risks, and reporting results that sometimes contradicted the clinical data or the previously reported results [
3,
4,
5,
8,
10,
11,
12]. However, a series of recent FEA studies [
3,
4,
5,
8,
10,
11,
12], comparing the different failure criteria, arguing the material-based type failure criteria selection, and correlating the MHP and ischemic risks with the biomechanical behavior of tooth, PDL, dental pulp, and NVB, reported Tresca failure criteria as providing more accurate results than that of the other failure criteria.
Tresca failure criteria state that failure occurs in a material when the absolute value of maximum shear stress reaches the shear stress at yielding and is consistent with the failure being independent of hydrostatic stress.
Clinical external resorption studies have reported that compression and pressure surfaces (i.e., stress distribution) were more prone to resorptive risks [
20], while the severity of the resorptive process (depth and extent) was correlated with the amount of applied force [
7,
20,
34]. Based on these observations, the need to respect the anatomical accuracy of the analyzed 3D models (the lacunae location is dependent on the anatomical morphology and curvatures of the tooth) becomes evident [
34]. Another report [
34] suggested the importance of the correlation between stress distribution, root morphology, and the resorptive lacunae, with lesser importance given to the amount of the applied force. This approach reinforces the concept that the shear stress induces in the root and coronal surfaces local pressures, producing localized resorptive lacunae, while in the dental pulp and PDL, various levels of ischemia are produced, triggering both the orthodontic movement, the resorptive process, and a further tissue loss. Practically, the resorptive process depends on the amount of applied force strictly correlated with the absorption–dissipation ability of the tooth and the exceeding of the 4.7 KPa of minimum hydrostatic pressure but without further exceeding the 16 KPa of the MHP, as well as the periodontal support level [
4,
5,
8,
12,
34]. Furthermore, the high absorption–dissipation ability of the tooth for internal shear stress was clinically reported through the prevalence of internal resorption vs. the external one [
1,
2,
6,
7,
14], and through FEA reports [
4,
5,
8,
12] about higher amounts of shear stresses reaching the PDL than those reaching the dental pulp and NVB.
FEA studies are the only methods to individually assess the stress distribution and display in living tissues with accurate results (as in the engineering field) if the requirements for the use of the criteria are met [
3,
4,
5,
8,
10,
11,
12,
21,
22]. The few studies [
7,
20,
28,
29,
30,
31,
34] that assessed the external root resorption by combining an in vivo–in vitro experiment and an FEA analysis of an intact periodontium, reported FEA results that did not accurately match the clinical report. Moreover, a non-internal resorption study was found probably due to the difficulty in the analysis of this type of lesion, despite its high reported prevalence of up to 51.1% [
2]. Thus, the FEA analysis employing a ductile materials failure criterion (maximum shear stress—Tresca [
4,
5,
8], more adequate for the study of dental tissues) was considered an adequate approach in the study of the internal resorption issue.
This FEA analysis aimed to investigate the areas more exposed to the risks of orthodontic internal resorption during 0–8 mm of periodontal breakdown under 0.6 N/60 gf and 1.2 N/120 gf and under various movements, as well as the absorption–dissipation ability of the tooth. Additionally, if there are significant differences between the biomechanical behavior of the two forces, they were also assessed.
2. Materials and Methods
The current FEA analysis represents a stepwise study of a more comprehensive research (clinical protocol nr.158/02.04.2018) of the biomechanical behavior of the tooth and surrounding support tissues under orthodontic movements during the horizontal periodontal breakdown [
3,
4,
5,
8,
10,
11].
This analysis involved a number of 324 simulations on eighty-one anatomically correct 3D models from nine patients (mean age of 29.81 ± 1.45 years, 4 males and 5 females, oral informed consent).
This research examined a large number of patients, but a rather restrictive inclusion criteria reduced their number to nine. These inclusion criteria were chosen for reducing the biomechanical uncertainties (i.e., intact mandibular arch, no malposition, intact teeth, no root canal endodontic treatment, no filling, non-inflamed periodontium, moderate/reduced bone loss in the cervical third, orthodontic treatment, regular periodic checks). The exclusion criteria were incomplete arches, malposition teeth, restored teeth, large amount of bone loss, and inflamed periodontium. Thus, the sample size was nine (nine patients; nine models/patient; a total of 81 3D models and 324 FEA simulations), as opposed to current FEA studies analyzing a sample size of one (one model of one patient and few FEA simulations) because of the 3D model creation. It must be emphasized that most of the previous FEA studies [
7,
9,
15,
16,
17,
20,
22,
23,
26,
27,
28,
29,
30,
31,
32,
33,
34] used for comparison with the herein results used a sample size of one (one patient and one model), with the exception of our earlier one [
3,
4,
5,
8,
10,
11] (a sample size of nine).
The starting point for the 3D models was the CBCT (cone beam computed tomography, ProMax 3DS, Planmeca, Helsinki, Finland, voxel size 0.075 mm) examination of the mandibular arch (i.e., premolars and molars). The manual image reconstruction was performed by a single practitioner employing the AMIRA 5.4.0 software (Visage Imaging Inc., Andover, MA, USA). On each radiological image/slice the anatomical components were identified (based on the Hounsfield grey shade units) and selected. Thus, the enamel, dentine, bracket, periodontal ligament, dental pulp neurovascular bundle, and cortical and trabecular bone were found and selected. Due to difficulties in identifying the cementum component and the similar physical properties with the dentine (
Table 1), the cementum was reconstructed as dentine (the radicular dentine/cementum component). All these components were merged in a 3D model (for each of the nine patients, e.g.,
Figure 1 and
Figure 2) with limited varied cervical third bone loss.
The periodontal ligament has an average thickness of 0.15–0.22 mm and was included in the apical third of the neurovascular bundle of the dental pulp. The first premolar and the two molars were replaced by cortical and trabecular bone. The missing bone and PDL were as much as possible closely anatomically reconstructed, obtaining nine models with an intact periodontium with the second lower premolar.
All models were then subjected to smoothing and refinement processes, obtaining 3D models with a total of 5.06–6.05 million C3D4 tetrahedral elements, 0.97–1.07 million nodes, and global element size of 0.08–0.116 mm (e.g.,
Figure 1 and
Figure 2). All models had surface irregularities since the reconstruction was manually conducted. However, the internal algorithm of the reconstruction and FEA software does not allow a further step when there are errors, anomalies, and/or irregularities that could interfere with the biomechanical behavior. Thus, despite the presence of surface irregularities, those are in non-essential areas, while the stress display areas are quasi-continuous. Moreover, the FEA software allows a mesh testing for evaluating the total number of errors and warnings, resulting in no errors and only a limited number of element warnings (e.g.,
Figure 1 and
Figure 2: 264 element warnings (representing 0.0043%) for the entire model of 6.05 million C3D4 elements; 63 element warnings (0.00677%) for the 930,023 elements of the tooth, bracket, and PDL; 26 element warnings (0.00459185%) for the 566,221 elements of the radicular dentine/cementum and coronal dentine; and 17 element warnings (0.0141469%) for the 120,168 elements of the enamel and bracket).
Each of the intact periodontium nine models was subjected to gradual horizontal PDL and bone reduction of 1 mm, simulating a horizontal periodontal breakdown process of 0–8 mm of loss, obtaining a total of 81 models.
The assumed boundary conditions were isotropy, homogeneity, and linear elasticity as in the other studies found in the scientific flow. Moreover, the assumptions were considered acceptable since under small loads (around 1 N/100 gf), the biomechanical movements are extremely small, and all tissues display linear elasticity.
The FEA analysis totaling 324 simulations was conducted using the ABAQUS6.13-1 software (Dassault Systèmes Simulia Corp., Maastricht, The Netherlands). The failure criteria were Tresca maximum shear stress specially designed for describing the biomechanical behavior of ductile resemblance non-homogeneous materials, considered to be more adequate for the study of dental tissues than other criteria [
8,
10,
11].
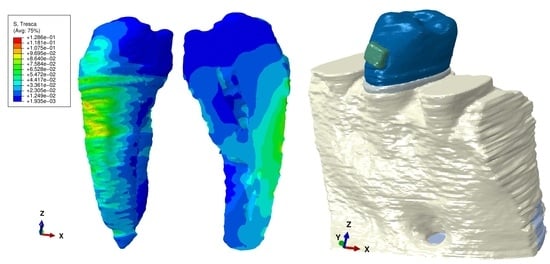
The applied forces at the bracket level (e.g.,
Figure 1) were 0.6 N/approx. 60 gf and 1.2 N/approx. 120 gf, simulating five orthodontic movements (extrusion, intrusion, tipping, rotation, and translation). Those forces were chosen not only because they are often used in clinical practice but also to establish correlations with previous analyses [
4,
5,
8] of PDL and dental pulp and NVB, thus improving the knowledge regarding the biomechanical behavior of teeth subjected to periodontal breakdown.
Figure 1.
Mesh model: (A) 2nd lower right premolar model with intact periodontium and applied vector for extrusion, (B) mesh model with 4 mm bone loss, (C) mesh model with 8 mm bone loss, (D) applied vector for intrusion, (E) applied vector for rotation, (F) applied vector for tipping, (G) applied vector for translation, (H) mesh model of radicular dentine/cementum and coronal dentine, (I) enamel and bracket components, (J) 2nd mandibular premolar with applied bracket, (K) section of tooth with pulp-camber and root canals.
Figure 1.
Mesh model: (A) 2nd lower right premolar model with intact periodontium and applied vector for extrusion, (B) mesh model with 4 mm bone loss, (C) mesh model with 8 mm bone loss, (D) applied vector for intrusion, (E) applied vector for rotation, (F) applied vector for tipping, (G) applied vector for translation, (H) mesh model of radicular dentine/cementum and coronal dentine, (I) enamel and bracket components, (J) 2nd mandibular premolar with applied bracket, (K) section of tooth with pulp-camber and root canals.
Figure 2.
Mesh model: (A) mesh model grid with intact periodontium and 63 elements warning for the tooth, bracket, and PDL, (B) 39 elements warnings for tooth and bracket, (C) model with no bone loss without the mesh grid, (D) tooth with bracket dental pulp and NVB without the mesh grid, (E) radicular dentine/cementum and coronal dentine components with NVB, (F) dental pulp and NVB.
Figure 2.
Mesh model: (A) mesh model grid with intact periodontium and 63 elements warning for the tooth, bracket, and PDL, (B) 39 elements warnings for tooth and bracket, (C) model with no bone loss without the mesh grid, (D) tooth with bracket dental pulp and NVB without the mesh grid, (E) radicular dentine/cementum and coronal dentine components with NVB, (F) dental pulp and NVB.
Table 1.
Elastic properties of materials.
Table 1.
Elastic properties of materials.
| Material | Young’s Modulus, E (GPa) | Poisson Ratio, ʋ | Refs. |
|---|
| Enamel | 80 | 0.33 | [3,4,5,8,10,11] |
| Dentine/Cementum | 18.6 | 0.31 | [3,4,5,8,10,11] |
| Pulp | 0.0021 | 0.45 | [3,4,5,8,10,11] |
| PDL | 0.0667 | 0.49 | [3,4,5,8,10,11] |
| Cortical bone | 14.5 | 0.323 | [3,4,5,8,10,11] |
| Trabecular bone | 1.37 | 0.3 | [3,4,5,8,10,11] |
| Bracket (Stainless Steel) | 190 | 0.265 | [3,4,5,8,10,11] |
The FEA supplied qualitative color-coded (projections of the shear stress areas) and quantitative results (average shear stress). The color-coded results were red-orange (maximum shear stress prone to high resorptive risks), yellow (high shear stress having a moderate to high risk of resorption), yellow-green (moderate resorption risks), and blue-green (reduced resorptive risks). These color-coded areas were displayed on both the entire tooth structure (including pulp and bracket) and on the radicular dentine/cementum and coronal dentine components (on both internal and external surfaces), analyzing the apical, middle, and cervical thirds and their sides/walls (vestibular, lingual, mesial, distal). The quantitative average shear stresses were correlated with the quantitative shears stress for PDL [
5] and dental pulp–NVB [
4] (under 0.6 N and 1.2 N of applied force) for assessing the ability of tooth structure to absorb–dissipate [
8] the stresses and highlight the areas more exposed to risk of resorption.
4. Discussion
This finite elements analysis assessed the internal surface resorption risks in the dentine/cementum and coronal dentine components employing the maximum shear stress criteria (to the best of our knowledge being the only one of this type). By simulating the conditions of a gradual horizontal periodontal breakdown, it was possible to study the influence of bone loss over the internal resorptive process during the five orthodontic movements and under 0.6 and 1.2 N.
Since no other studies regarding these issues were found, the correlation of the results herein was performed with the results of external [
7,
20,
28,
29,
30,
31,
34] and internal [
9] resorption studies, due to the close correlation between the external and internal surface resorptive processes [
1,
2,
3,
6,
7,
14,
19,
20,
34].
The maximum shear stress criteria were able to provide an accurate display of the correlated localized areas of maximum (red-orange) and high (yellow and yellow-green) stress on both the external and internal radicular dentine/cementum and coronal dentine surfaces. These areas are prone to high and moderate risks of the resorptive process, since the resorption seems to be the result of localized stress concentrations [
18] due to the force appliance, anatomical morphology, and movement closely correlated with individual susceptibility [
1,
2,
3,
6,
7,
14,
19,
20,
34].
When comparing the results herein with previous studies [
7,
20,
28,
29,
30,
31,
34], the first issue that arises is related to the different failure criteria employed in the FEA analysis, which could significantly interfere with the accuracy of the results [
8,
10,
11,
21]. The FEA was originally developed for the engineering field, studying materials with micro-architectures less complicated than human tissues and subjected to excessive amounts of force. Each failure criterion was mathematically designed to reproduce and describe the biomechanical behavior of a certain type of material, with limited but visible differences under small, applied forces and movements, but significantly increasing with the amount of force and the amplitude of the movements [
8,
10,
11].
Previous external surface resorption studies [
7,
20,
28,
29,
30,
31,
34] largely employed the hydrostatic pressure criterion, specially designed for liquids and gas (i.e., a physical condition where there are no shear stresses), while the analyzed tissues (tooth and periodontium) resemble more ductile materials (with a certain brittle flow mode) that suffer from important shear stresses [
3,
4,
5,
8,
10,
11,
12,
23,
26,
27]. The criteria for ductility are the von Mises overall stress (for homogenous materials) and Tresca maximum shear stress (for non-homogenous materials) [
3,
4,
5,
8,
10,
11,
12]. FEA comparative studies reported for dental tissues that Tresca and von Mises are adequate, with Tresca providing more accurate results [
3,
4,
5,
8,
10,
11,
12]. The main issues regarding the difference between the hydrostatic criteria studies [
7,
20,
28,
29,
30,
31,
34] and the Tresca criteria were related to the qualitative stress display, with a more accurate and localized stress area for Tresca [
3,
4,
5,
8,
10,
11,
12]. There are also notable quantitative differences, with the hydrostatic studies providing various amounts of stress that sometimes contradict the clinical data [
28,
29] and/or reporting optimal forces varying from one study to another for the same tooth, movement, and boundary conditions [
15,
16,
17], in contrast to reports by Proffit et al. [
13].
The present study (by employing the adequate material-based failure criterion), analyzing the tooth structure, radicular dentine/cementum, and coronal dentine, showed a shear stress display correlated with the bone loss level for both the external and internal prone-to-resorption areas. The external surface radicular dentine/cementum and coronal dentine displayed higher resorptive risks when compared with the internal surface (external surface red-orange and yellow areas vs. internal surface yellow and yellow-green areas), in line with reports regarding the absorption–dissipation ability of the tooth structure [
3,
4,
5,
8,
10,
11,
12,
23,
24,
25,
26,
27]. No significant visible qualitative differences between the 0.6 N and 1.2 N color-coded display areas (about both external and internal surfaces) were seen, so it seems that the only difference consists in a doubling of the amount of quantitative stress display (
Table 2).
The resorptive risks seemed to increase with the progression of the bone loss, since most of the maximum shear stress areas of the external surface were displayed around the bone support level and with a visible correspondence of stress display on the internal surface (
Figure 3,
Figure 4,
Figure 5,
Figure 6 and
Figure 7).
Internal surface moderate resorptive risks (yellow and yellow-green) were visible in both the root canals and radicular and coronal pulp chamber. The biomechanical color-coded display of shear stress seemed to change along with the bone loss for the coronal dentine vs. the radicular dentine/cementum (
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7 and
Figure 8). If, in the intact periodontium, the color-coded yellow areas are present, especially in the vestibular side/wall of the pulp chamber and cervical third of the radicular dentine/cementum (blue-green in middle and apical third), with the progression of periodontal breakdown, the stress decreases in the pulp chamber (blue-green) and increases (yellow and yellow-green) in the radicular dentine in the middle and apical third, for all five movements and forces (
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7 and
Figure 8).
Based on the color-coded stress display and quantitative results, the rotational and translational movements seem to be more prone to resorptive risks, while the intrusion and extrusion are less predisposed. It seems that the internal resorptive risks increase with bone loss if the applied force remains unchanged as in the intact periodontium.
It must be emphasized that the two forces 0.6 N and 1.2 N are considered light forces, while, biomechanically, if the amount of force further increases, the results could also change [
2,
3,
4,
5,
6,
8,
10,
11,
13,
14], since around 1 N of applied force and under extremely small/reduced displacements/movements, all tissues display linear elasticity (i.e., the higher the force, the more significant the changes appear in the biomechanical behavior). The selection of the amount was chosen due to their frequent use in clinical practice and their employment in previous FEA simulations focusing on PDL and dental pulp and NVB [
4,
5]. These FEA studies [
4,
5] reported that 1.2 N could be safely used in an intact periodontium for all movements, while at 8 mm of loss, 0.6 N should not be exceeded.
Only one study assessing the internal resorptive issues was found. Thus, Aslan et al. [
9] (300 N occlusal force buccal side oriented, von Mises, single idealized mandibular premolar with root canal filling with gutta/MTA, intact periodontium, 271,837 tetrahedral elements, and 414,930 nodes) reported higher buccal stress than lingual in the cervical and middle thirds of the root, with external surface maximum red-orange overall stress of 74.32–107 MPa (approx. 74,322–107,000 KPa), and high yellow stress of 57.8–83.1 MPa (approx. 57,800–83,100 KPa) and internal surface stress maximum red-orange of 150.5 MPa (approx. 150,500 KPa) cervical, and 381.1 MPa (approx. 381,100 KPa) middle thirds. These results were quantitatively significantly higher than the 29–73.1 MPa reported as maximum shear stress for dentine [
24], presuming extremely high risks of resorptive processes, with a large extension on both external and internal surfaces, which would be unusual in daily practice.
The main differences between Aslan et al. [
9] and the 3D model study, herein, are related to the fact that the internal resorptions were artificially simulated as cavities of 1.8–3.8 mm in diameter (while the remaining thickness of dentine was 1 mm) with an unusual topographic display, which significantly modifies the biomechanical absorption–dissipation ability of the dental structure correlated with the anatomically inaccurate 3D models (14 times fewer tetrahedral elements).
In our study, in the intact periodontium, the quantitative stress for 0.6 N of intrusion was reported as being 73.5–109.4 KPa on the radicular surface of the tooth structure, and 44.1–54.7 KPa on the internal surface of the radicular dentine/cementum. The qualitative results of the external stress distribution showed a similar display area on the vestibular and lingual sides of the premolar for stress (but a different color-coded display).
It must be emphasized that for more than 1 N of force, the linear elasticity premises are no longer correct and adequate for dental tissue biomechanical behavior. Moreover, von Mises failure criteria (overall stress) were designed for ductile homogenous structures, and tooth and surrounding periodontium do not meet these requirements. Only Tresca (maximum shear stress) criteria were designed for non-homogenous materials, and the linear elasticity assumptions became more and more imprecise as the applied force increased. The difference between the two amounts of force (300 N vs. 0.6 N) also represents two different functional circumstances, maximum masticatory bite load (e.g., 20–120 N average bite load [
22]) vs. current practical orthodontic loads, with significantly different biomechanical behavior and tissues reactions (endodontically root canal-treated premolar vs. intact tooth, in our simulation).
Ordinola-Zapata et al. [
22], in a review study, reported the quasi-general use of a mix of brittle (maximum principal stress) and ductile (von Mises) failure criteria in the FEA studies of dentine (found in the current research flow), as well as their opinion that dentine is a brittle solid, in contrast to the clinical knowledge, FEA, and in vitro reports about obvious ductility [
3,
4,
5,
8,
10,
11,
12,
23,
24,
25,
26,
27].
From the biomechanical point of view, brittle materials do not show a significant absorption–dissipation ability, rather the energy is not dissipated, and the stress wave propagates determining cracks and fractures (due to limited/no ability of deformation). Moreover, despite the biomechanically acknowledged and reported [
8,
10,
23,
26,
27] ability of dentine and dentine/cementum to absorb–dissipate stresses (specific to ductile), no studies have approached this issue except ours [
8]. Thus, correlation and relationships with an earlier study [
4] of our group focusing on the dental pulp–NVB stress (possible because of the employment of similar boundary conditions and physical properties) allowed the quantification of the ability of the dentine to absorb and dissipate the stress reaching the dental pulp (
Table 3). In both intact and reduced periodontium, from a total of 0.6 and 1.2 N, the dentine absorbed about 98.1–99.97% of the stress determined by the applied orthodontic force, and only 0.46–2.38% reached the pulpal tissue, which is in line with the expected biomechanical behavior (
Table 3). Moreover, this correlation also considered the MHP value that was not exceeded either in PDL [
5] nor in pulp–NVB [
4] (to reduce the ischemic risks and further periodontal loss) as being as accurate as possible and closer to the reported clinical data.
Field et al. [
31], considering the brittleness of dental tissues and employing multiple failure criteria (von Mises, Hydrostatic pressure, and Maximum S1 and Minimum S3 Principal Stress) and 0.35 N/0.5 N of tipping over a 3D mandibular model (23,565–32,812 elements, global element size 1.2 mm, and incisor–canine–premolar), reported that the apical third hydrostatic pressure was two times higher than that of the MHP, and S1 and S3 stresses for PDL were fourteen times higher than that of the MHP, implying a significative number of resorptive processes for the periodontium and root, and extended tissue necrosis for a light orthodontic force, in contrast to clinical knowledge [
2,
3,
4,
5,
6,
8,
10,
11,
13,
14]. The main issues in this report (as for most FEA studies) were the material-type assumptions and the anatomical accuracy of the employed models [
3,
4,
5,
8,
10,
11,
21].
The FEA resorption studies [
7,
20,
28,
29,
30,
34] related to orthodontic external root resorption in an intact periodontium considered the maximum hydrostatic pressure criterion for their analyses. These reports, which assessed the accuracy of FEA in displaying areas of high-stress resorptive risks and were correlated with the in vivo–in vitro results, found resorptive lacunae, with visible discrepancies between the FEA and clinical data (i.e., the FEA results usually identified the side where the resorption could appear and not the localized points of high stress and pressure, as expected [
18]).
Only Zhong et al.’s [
7] study showed, for an intact periodontium, a correlated image between the in vivo–in vitro results and FEA external root resorption lacunae (with variable diameter, positions, and extensions varying with the tooth being analyzed), displaying as a pattern the recurrent location of the side of the root (in agreement with herein study). Thus, the idea related to the role of anatomical particularities and individual reactivity in both external and internal resorption seems acceptable and confirmed (but with a need for further studies), as well as the unpredictability of resorption due to the variability of the locations of the points of pressure. Nevertheless, the problem of not accurately identifying the point of pressure but only the side of the root was also clearly visible in this correlation [
7].
The Tresca criteria results seem to resemble more those provided by the clinical data [
7,
20,
28,
29,
34], while the differences between our maximum shear stress and hydrostatic stress [
7,
28,
29] simulations come both from the inadequate selection of the failure criteria and various applied boundary conditions [
8,
10,
11].
Ordinola-Zapata et al. [
22], in a review study regarding the employment of the FEA method in the study of treated teeth, emphasized the importance of the correct input data and boundary conditions. The anatomical correctness of the analyzed models is essential, especially in the study of internal and external orthodontic resorption, since the process is highly dependent on the individualities of the root morphology [
18] and tissue’s anatomical architecture [
1,
2,
6,
7,
14,
19,
20,
34]. Thus, a mesh with a high number of elements and nodes and a reduced global element size will provide more anatomical accuracy (herein, 5.06–6.05 million C3D4 tetrahedral elements with a global element size of 0.08–0.116 mm vs. Aslan et al.’s [
9] 271,837 tetrahedral elements, 414,930 nodes anatomically idealized premolar, or Field et al.’s [
31] mandibular incisor–canine–premolar model of 23,565–32,812 elements, with a global element size 1.2 mm).
The boundary conditions are related to the applied assumptions of anatomical micro-architecture (isotropy, homogeneity, and linear elasticity vs. anisotropy, non-homogeneity, and non-linear elasticity). The ductile failure criteria are specially designed for homogenous (von Mises) and non-homogenous (Tresca) materials, and hydrostatic pressure for liquids (no shear stress).
The linear elasticity assumption is correct only if there are limited movements/displacements and up to 1 N of applied force (as in herein), while as the applied force increases, the non-linear behavior of the anatomical tissues influences the results and becomes increasingly visible (discrepancies between the FEA and clinical data). There are FEA studies [
30,
32,
33] that have analyzed the differences in linearity vs. non-linearity for PDL, reporting on under 1 N of applied force differences of up to 20% (i.e., reduction for non-linearity), and finding that non-linear hydrostatic is the only adequate criteria for PDL. However, the employed failure criteria were that of maximum and minimum principal [
32,
33] stress (for brittle solids), despite the PDL being of ductile resemblance, von Mises (homogenous ductile), and hydrostatic pressure (liquids/gas) [
30], without arguing their choice of criteria; thus, their results should be considered with care.
Isotropic material displays the same properties in all directions, while anisotropy represents a change in properties of the different directions/plans. The difference between these two conditions is extremely small under small loads and movements, but significant if those conditions change. Moreover, almost all FEA studies [
9] assumed isotropy, linear elasticity, homogeneity, and perfectly bonded surfaces for all components of the models, except the PDL, where the Ogden hyperelastic model [
15,
16,
17] was applied. The Odgen model was specially designed to describe the non-linear stress–strain behavior of rubber and polymers (non-linearly elastic, isotropic, and incompressible) and some biological tissues (PDL did not accurately match the model due to its specific anatomical micro-architecture).
Thus, Wu et al. [
15,
16,
17], using the hydrostatic stress criteria and assuming homogeneity, isotropy, and linear elasticity (except for PDL–Ogden non-linear hyperelastic) in an intact periodontium, reported an optimal force of 0.28–3.31 N for the intact periodontal ligament of canine, premolar, and lateral incisive, but with significant differences from one study to another for the same tooth (e.g., canine: rotation 1.7–2.1 N [
17] and 3.31 N [
15]; extrusion 0.38–0.4 N [
17] and 2.3–2.6 N [
16]; premolar: rotation 2.8–2.9 N [
15]), and higher than the clinically accepted light forces of 0.5–1 N/approx. 50–100 gf [
2,
4,
5,
6,
8,
10,
11,
13,
14].
Another critical issue that could interfere with the results of an FEA simulation is related to the used sample size. In the herein simulations, the sample size was nine (nine models originated from nine patients, totaling eighty-one models and 324 simulations), in contrast to the above-mentioned FEA analyses with a sample size of one (one patient and one model). It is accepted that for increasing the accuracy of results, the higher the number of analyzed models, the better the accuracy. Nevertheless, the FEA studies employed only one 3D model (except for our study) due to difficulties in the reconstruction process. The reconstruction process could be performed manually (better anatomical accuracy but extremely difficult and time-consuming, as herein) or automated (less accuracy but also less difficult and time-consuming). The herein models had 28–184 times more elements and a global element size ten times lower than the above-mentioned FEA studies.
Our herein FEA analysis, despite the limited correlation with other internal resorption studies (no orthodontic internal resorption studies were found), showed that the localized areas of potential resorptive risks seemed to vary more with the movement and bone loss level, and less with the amount of applied force. A possible explanation is the fact that both employed forces were light and the movements/displacements were extremely small, while other FEA studies reported a change in the diameter and depth of the resorptive lacunae with the increase in force [
7,
20,
34]. Such a variation could also be possible for our models if a higher force were applied. Nevertheless, previous FEA studies reported that 1.2 N could be safely applied in both an intact periodontium and 0.6 N in 8 mm bone loss; thus, a higher force would increase the risks of further periodontal loss [
2,
3,
4,
5,
6,
8,
10,
11,
13,
14]. Moreover, our study prefers the hypothesis that the individuality of each patient correlated with the root morphology, and also plays an important part in the location of the displayed high-pressure areas [
18], in agreement with other studies [
1,
2,
6,
7,
14,
19,
20,
34].
In the absence of other FEA studies, the maximum shear stress criteria seem to offer the highest accuracy for potential resorptive areas, while simulations of external–internal resorption should be developed in a reduced periodontium (often encountered among orthodontic patients). In addition to the above-addressed limits (i.e., failure criteria, boundary conditions, material type, amount of force, and mesh model), it must be emphasized that the FEA analysis, despite being the only possible method for this type of study, does not accurately reproduce clinical conditions; thus, correlations with in vivo–in vitro reports are needed.