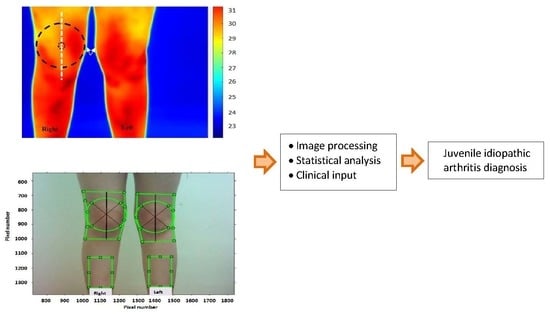
Thermal and Visual Imaging to Assist with Juvenile Idiopathic Arthritis Examination of the Knees
Abstract
:1. Introduction
- Devising a consistent method to extract the region of interest (ROI) centered on the knee and a procedure to analyze its temperature.
- The analysis of knee temperature based on the sub-grouping of the participants into one knee, both knees and neither knee with active inflammation.
- Correspondence analysis between thermal and visual (colour) imaging in JIA participants.
- Correspondence analysis of thermal and visual imaging to the clinician’s assessment in identifying inflamed joints in participants with JIA.
2. Methods
2.1. Ethical Approvals
2.2. Recruitment
2.3. Medical Examinations of Participants
2.4. Thermal and Visual Image Recordings
2.5. Image Processing Procedures
2.5.1. Thermal Image Processing Procedure
2.5.2. Visual Image Processing
- i.
- The direct comparison of the colours of the right and left knees in each participant. This was not used as the method assumed that there was no baseline colour difference between the knees. Knees unaffected by arthritis may not have an identical skin colour due to factors such varying exposure to sun light etc.
- ii.
- The comparison of the colour of each knee with a reference region taken from under the same knee. In this method, any baseline colour difference would have been considered. This method was also not used as it meant that the two knees would have had different references, making the comparison of results inaccurate.
- iii.
- The method implemented compared the colours of the knees with the average of the reference regions taken under each knee. In this method, a common reference was used for both knees and the sources of error in methods (i) and (ii) were dealt with. The reference region was mid-way between the ankle and the knee. The method, however, assumed that arthritis had not affected the colour of the reference region. Figure 3 shows the reference regions under the knees manually cropped by displaying the visual image on the screen. The reference regions taken from the left and right legs had the same size and were averaged to produce a common reference for both knees.
- The histograms of each knee’s ROI’s colour components (i.e., red, green and blue) and those for the common reference were obtained. The histogram for a colour component indicated the frequency of the occurrence of shade of colour for the related component. The cropped regions for the knees appeared on a square frame, with areas not covered by the selected region appearing black as shown Figure 4a,b. Therefore, this black colour was excluded from the histograms as it represented the image background after it had been cropped.
- For each knee, the Euclidian distance between each colour component histogram and common reference region was obtained. The resulting three distances were then averaged to obtain a single distance measure quantifying the colour difference for the knee. The knee with a larger distance from the reference region was considered to have a greater colour difference.
3. Results and Discussion
- Group I: participants with one inflamed knee joint.
- Group II: participants with both knees actively inflamed.
- Group III: participants with neither knee joint inflamed.
3.1. Correspondence between Thermal Imaging and Clinical Evaluation
3.1.1. Group I Participants
3.1.2. Group II Participants
3.1.3. Group III Participants
3.2. Correspondence between Visual Imaging, Thermal Imaging and Clinician’s Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ravelli, A.; Martini, A. Juvenile idiopathic arthritis. Lancet 2007, 369, 767–778. [Google Scholar] [CrossRef] [Green Version]
- Prakken, B.; Albani, S.; Martini, A. Juvenile idiopathic arthritis. Lancet 2011, 377, 2138–2149. [Google Scholar] [CrossRef] [Green Version]
- Boros, C.; Whitehead, B. Juvenile idiopathic arthritis. Aust. Fam. Physician 2010, 39, 630–636. [Google Scholar]
- Chang, H.J.; Burke, A.E.; Glass, R.M. Juvenile Idiopathic Arthritis. JAMA 2010, 303, 1328. [Google Scholar] [CrossRef] [Green Version]
- Wallace, C.; Giannini, E.H.; Spalding, S.J.; Hashkes, P.J.; O’Neil, K.M.; Zeft, A.S.; Szer, I.S.; Ringold, S.; Brunner, H.I.; Schanberg, L.E.; et al. Trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis. Arthritis Rheum. 2011, 64, 2012–2021. [Google Scholar] [CrossRef] [Green Version]
- Baildam, E. A complementary on TREAT: The trial of early aggressive drug therapy in juvenile idiopathic arthritis. BMC Med. 2012, 10, 59. Available online: https://bmcmedicine.biomedcentral.com/track/pdf/10.1186/1741-7015-10-59 (accessed on 24 October 2019). [CrossRef] [Green Version]
- Ravelli, A.; Consolaro, A.; Horneff, G.; Laxer, R.M.; Lovell, D.J.; Wulffraat, N.M.; Akikusa, J.D.; Al-Mayouf, S.M.; Antón, J.; Avčin, T.; et al. Treating juvenile idiopathic arthritis to target: Recommendations of an international task force. Ann. Rheum. Dis. 2018, 77, 819–828. [Google Scholar] [CrossRef]
- Giancane, G.; Ruperto, N. Paediatric Rheumatology International Trials Organisation (PRINTO) Treatment of juvenile idiopathic arthritis. Curr. Opin. Rheumatol. 2019, 31, 428–435. [Google Scholar] [CrossRef]
- VersusArthritis. Defying Arthritis Together. Available online: https://www.versusarthritis.org/ (accessed on 25 October 2019).
- Foster, H.E.; Kay, L.J.; Friswell, M.; Coady, D.; Myers, A. Musculoskeletal screening examination (pGALS) for school-age children based on the adult GALS screen. Arthritis Rheum. 2006, 55, 709–716. [Google Scholar] [CrossRef]
- Selaas, O.; Nordal, H.H.; Halse, A.-K.; Brun, J.G.; Jonsson, R.; Brokstad, K.A. Serum Markers in Rheumatoid Arthritis: A Longitudinal Study of Patients Undergoing Infliximab Treatment. Int. J. Rheumatol. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Giancane, G.; Consolaro, A.; Lanni, S.; Davì, S.; Schiappapietra, B.; Ravelli, A. Juvenile Idiopathic Arthritis: Diagnosis and Treatment. Rheumatol. Ther. 2016, 3, 187–207. [Google Scholar] [CrossRef] [Green Version]
- Ramos, P.C.; Ceccarelli, F.; Jousse-Joulin, S. Role of ultrasound in the assessment of juvenile idiopathic arthritis. Rheumatology 2012, 51, vii10–vii12. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.; Uleryk, E.; Doria, A.S. Evidence-Based Outcomes of Studies Addressing Diagnostic Accuracy of MRI of Juvenile Idiopathic Arthritis. Am. J. Roentgenol. 2009, 192, 1209–1218. [Google Scholar] [CrossRef]
- Ostergaard, M.; Edmonds, J.; McQueen, F.; Peterfy, C.; Lassere, M.; Ejbjerg, B.; Bird, P.; Emery, P.; Genant, H.; Conaghan, P.G. An introduction to the EULAR–OMERACT rheumatoid arthritis MRI reference image atlas. Ann. Rheum. Dis. 2005, 64, i3–i7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamanti, A.P.; Navarini, L.; Messina, F.; Markovic, M.; Arcarese, L.; Basta, F.; Meneguzzi, G.; Margiotta, D.P.E.; Laganà, B.; Afeltra, A.; et al. Ultrasound detection of subclinical synovitis in rheumatoid arthritis patients in clinical remission: A new reduced-joint assessment in 3 target joints. Clin. Exp. Rheumatol. 2018, 36, 984–989. [Google Scholar]
- Hemke, R.; Maas, M.; Van Veenendaal, M.; Dolman, K.M.; Van Rossum, M.A.; Berg, J.M.V.D.; Kuijpers, T.W. Contrast-enhanced MRI compared with the physical examination in the evaluation of disease activity in juvenile idiopathic arthritis. Eur. Radiol. 2013, 24, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Hemke, R.; Lavini, C.; Nusman, C.M.; Berg, J.M.V.D.; Dolman, K.M.; Schonenberg-Meinema, D.; Van Rossum, M.A.; Kuijpers, T.W.; Maas, M. Pixel-by-pixel analysis of DCE-MRI curve shape patterns in knees of active and inactive juvenile idiopathic arthritis patients. Eur. Radiol. 2014, 24, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Spalding, S.J.; Kwoh, C.K.; Boudreau, R.; Enama, J.; Lunich, J.; Huber, D.F.; Denes, L.; Hirsch, R. Three-dimensional and thermal surface imaging produces reliable measures of joint shape and temperature: A potential tool for quantifying arthritis. Arthritis Res. Ther. 2008, 10, R10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerkvaleekul, B.; Jaovisidha, S.; Sungkarat, W.; Chitrapazt, N.; Fuangfa, P.; Ruangchaijatuporn, T.; Vilaiyuk, S. The comparisons between thermography and ultrasonography with physical examination for wrist joint assessment in juvenile idiopathic arthritis. Physiol. Meas. 2017, 38, 691–700. [Google Scholar] [CrossRef]
- Hawley, D.; Offiah, A.C.; Burke, D. PP10. Evaluation of skin temperature using liquid crystal and infrared thermometers in children attending specialist paediatric rheumatology clinics. Rheumatology 2015, 54. [Google Scholar] [CrossRef] [Green Version]
- Alkali, A.H.; Saatchi, R.; Elphick, H.; Burke, D. Thermal image processing for real-time non-contact respiration rate monitoring. IET Circuits Devices Syst. 2017, 11, 142–148. [Google Scholar] [CrossRef]
- Owen, R.; Ramlakhan, S.; Saatchi, R.; Burke, D. Development of a high-resolution infrared thermographic imaging method as a diagnostic tool for acute undifferentiated limp in young children. Med. Boil. Eng. 2017, 56, 1115–1125. [Google Scholar] [CrossRef] [Green Version]
- De Salis, A.F.; Saatchi, R.; Dimitri, P. Evaluation of high resolution thermal imaging to determine the effect of vertebral fractures on associated skin surface temperature in children with osteogenesis imperfecta. Med. Boil. Eng. 2018, 56, 1633–1643. [Google Scholar] [CrossRef]
- Borojevic, N.; Kolaric, D.; Grazio, S.; Grubisic, F.; Antonini, S.; Alelexandra, I. Thermography hand temperature distribution in rheumatoid arthritis and osteoarthritis. Period. Biol. 2011, 113, 445–448. [Google Scholar]
- Gabrhel, J.; Popracova, Z.; Tauchmannova, H.; Chvojka, Z. The connection of thermographic and musculoskeletal ultrasound examinations of the syndrome of painful knee. Rheumatologica 2012, 26, 1–21. [Google Scholar]
- Ilowite, N.T.; A Walco, G.; Pochaczevsky, R. Assessment of pain in patients with juvenile rheumatoid arthritis: Relation between pain intensity and degree of joint inflammation. Ann. Rheum. Dis. 1992, 51, 343–346. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, B.M.; Lesch, M.; Brammer, D.; Bove, S.E.; Thiel, M.; Kilgore, K.S. Use of a portable thermal imaging unit as a rapid, quantitative method of evaluating inflammation and experimental arthritis. J. Pharmacol. Toxicol. Methods 2008, 57, 169–175. [Google Scholar] [CrossRef]
- Brenner, M.; Braun, C.; Oster, M.; Gulko, P.S. Thermal signature analysis as a novel method for evaluating inflammatory arthritis activity. Ann. Rheum. Dis. 2005, 65, 306–311. [Google Scholar] [CrossRef] [Green Version]
- De Silva, M.; Kyle, V.; Hazleman, B.; Salisbury, R.; Thomas, P.P.; Wraight, P. Assessment of inflammation in the rheumatoid knee joint: Correlation between clinical, radioisotopic, and thermographic methods. Ann. Rheum. Dis. 1986, 45, 277–280. [Google Scholar] [CrossRef] [Green Version]
- Salisbury, R.S.; Parr, G.; De Silva, M.; Hazleman, B.L.; Page-Thomas, D.P. Heat distribution over normal and abnormal joints: Thermal pattern and quantification. Ann. Rheum. Dis. 1983, 42, 494–499. [Google Scholar] [CrossRef] [Green Version]
- Lasanen, R.; Piippo-Savolainen, E.; Remes-Pakarinen, T.; Kröger, L.; Heikkilä, A.; Julkunen, P.; Karhu, J.; Töyräs, J. Thermal imaging in screening of joint inflammation and rheumatoid arthritis in children. Physiol. Meas. 2015, 36, 273–282. [Google Scholar] [CrossRef]
- Snekhalatha, U.; Rajalakshmi, T.; Gopikrishnan, M.; Gupta, N. Computer-based automated analysis of X-ray and thermal imaging of knee region in evaluation of rheumatoid arthritis. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2017, 231, 1178–1187. [Google Scholar] [CrossRef]
- Jin, C.; Yang, Y.; Xue, Z.; Liu, K.; Liu, J. Automated analysis method for screening knee osteoarthritis using medical infrared thermography. J. Med. Biol. Eng. 2013, 33, 471–477. [Google Scholar] [CrossRef]
- Pincus, T. In the clinic. Rheumatoid arthritis. Ann. Intern. Med. 2010, 153, ITC1-15. [Google Scholar] [CrossRef]
- E Petty, R.; Southwood, T.R.; Manners, P.; Baum, J.; Glass, D.N.; Goldenberg, J.; He, X.; Maldonado-Cocco, J.; Orozco-Alcala, J.; Prieur, A.-M.; et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J. Rheumatol. 2004, 31, 390–392. [Google Scholar]
- Foster, H.E.; Jandial, S. pGALS–paediatric Gait Arms Legs and Spine: A simple examination of the musculoskeletal system. Pediatr. Rheumatol. 2013, 11, 44. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.P. Fast template matching. In Proceedings of the Vision Interface 95, Canadian Image Processing and Pattern Recognition Society, Quebec City, QC, Canada, 15–19 May 1995; pp. 120–123. Available online: http://scribblethink.org/Work/nvisionInterface/vi95_lewis.pdf (accessed on 24 October 2019).
- Munsayac, F.E.T.; Alonzo, L.M.B.; Lindo, D.E.G.; Baldovino, R.G.; Bugtai, N.T. Implementation of a normalized cross-correlation coefficient-based template matching algorithm in number system conversion. In Proceedings of the IEEE 9th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment and Management (HNICEM), Manila, Philippines, 1–3 December 2017. Corpus 39411265. [Google Scholar] [CrossRef]
- Yap, B.W.; Sim, C.H. Comparisons of various types of normality tests. J. Stat. Comput. Simul. 2011, 81, 2141–2155. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Ward, O.; Nwaizu, H.; Saatchi, R.; Ramlakhan, S.; Hawley, D. P36 Does thermal imaging correlate with musculoskeletal examination in the identification of inflamed joints in children and young people with juvenile idiopathic arthritis? A prospective diagnostic accuracy study. Rheumatology 2018, 57, 038. [Google Scholar] [CrossRef] [Green Version]
- Reed, C.; Saatchi, R.; Burke, D.; Ramlakhan, S. Infrared thermal imaging as a screening tool for paediatric wrist fractures. Med. Boil. Eng. 2020, 1–15. [Google Scholar] [CrossRef]
- Colebatch-Bourn, A.N.; Edwards, C.J.; Ramos, P.C.; D’Agostino, M.-A.; Hemke, R.; Jousse-Joulin, S.; Maas, M.; Martini, A.; Naredo, E.; Østergaard, M.; et al. EULAR-PReS points to consider for the use of imaging in the diagnosis and management of juvenile idiopathic arthritis in clinical practice. Ann. Rheum. Dis. 2015, 74, 1946–1957. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, D.S. Juvenile idiopathic arthritis: Diagnosis and differential diagnosis. Korean J. Pediatr. 2010, 53, 931–935. [Google Scholar] [CrossRef] [Green Version]
- Manzoni, S.M.; Malattia, C.; Lanni, S.; Ravelli, A. Advances and challenges in imaging in juvenile idiopathic arthritis. Nat. Rev. Rheumatol. 2012, 8, 329–336. [Google Scholar] [CrossRef]
- Jangjit, S.; Ketcham, M. A New Wavelet Denoising Method for Noise Threshold. Eng. J. 2017, 21, 141–155. [Google Scholar] [CrossRef] [Green Version]
- Saatchi, R.; Gibson, C.; Rowe, J.W.K.; Allan, E.M. Adaptive multiresolution analysis based evoked potential filtering. IEE Proc. Sci. Meas. Technol. 1997, 144, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Ouahabi, A.; Castanié, F. Signal and image multiresolution analysis. In Ebook; ISTE: London, UK; Hoboken, NJ, USA, 2012. [Google Scholar]
- Rabbani, H.; Nezafat, R.; Gazor, S. Wavelet-Domain Medical Image Denoising Using Bivariate Laplacian Mixture Model. IEEE Trans. Biomed. Eng. 2009, 56, 2826–2837. [Google Scholar] [CrossRef]
- Starck, J.-L.; Candes, E.; Donoho, D. The curvelet transform for image denoising. IEEE Trans. Image Process. 2002, 11, 670–684. [Google Scholar] [CrossRef] [Green Version]
- Po, D.D.Y.; Do, M.N. Directional multiscale modeling of images using the contourlet transform. IEEE Trans. Image Process. 2006, 15, 1610–1620. [Google Scholar] [CrossRef] [Green Version]









| Parameter | Details |
|---|---|
| Mean age (standard deviation) (years) | 11.2 (std: 2.3) |
| Sex | Male: 9 (std: 45%), Female: 11 (std: 55%) |
| Clinician assessment at time of thermal imaging |
|
| Medication for the 16 participants with active knee arthritis at the time of examination |
|
| Participant | JIA Diagnosis | Knee Side | Clinical Assessment | ||
|---|---|---|---|---|---|
| Abnormal Warmth | Swollen | Restricted Movement | |||
| 1 | Extended Oligoarticular: | Right | No | No | No |
| Left | Yes | Yes | Yes | ||
| 2 | Polyarticular: | Right | No | Yes | No |
| Left | No | Yes | No | ||
| 3 | Oligoarticular: | Right | Yes | Yes | Yes |
| Left | No | No | No | ||
| 4 | Polyarticular: | Right | No | No | No |
| Left | No | No | No | ||
| 5 | Oligoarticular: | Right | Yes | Yes | Yes |
| Left | No | No | No | ||
| 6 | Extended Oligoarticular: | Right | Yes | Yes | Yes |
| Left | No | No | No | ||
| 7 | Extended Oligoarticular: | Right | Yes | Yes | Yes |
| Left | Yes | Yes | Yes | ||
| 8 | Oligoarticular: | Right | Yes | Yes | Yes |
| Left | Yes | Yes | Yes | ||
| 9 | Extended Oligoarticular: | Right | No | No | No |
| Left | No | Yes | Yes | ||
| 10 | Oligoarticular: | Right | No | Yes | No |
| Left | No | No | No | ||
| 11 | Oligoarticular: | Right | No | No | No |
| Left | Yes | Yes | Yes | ||
| 12 | Oligoarticular: | Right | No | No | No |
| Left | No | Yes | No | ||
| 13 | Polyarticular: | Right | No | No | No |
| Left | No | No | No | ||
| 14 | Extended Oligoarticular: | Right | Yes | No | No |
| Left | Yes | No | No | ||
| 15 | Oligoarticular: | Right | Yes | Yes | Yes |
| Left | Yes | No | No | ||
| 16 | Oligoarticular: | Right | Yes | Yes | No |
| Left | Yes | Yes | No | ||
| 17 | Oligoarticular: | Right | Yes | Yes | Yes |
| Left | Yes | Yes | Yes | ||
| 18 | Oligoarticular: | Right | No | No | No |
| Left | No | No | No | ||
| 19 | Polyarticular: | Right | Yes | Yes | Yes |
| Left | Yes | Yes | Yes | ||
| 20 | Oligoarticular: | Right | No | No | No |
| Left | No | No | No | ||
| P | Temperature Obtained from Thermal Imaging (TI) (°C) | TI Based Assessment of the Knees’ ROIs | Clinician Assessment of the Knees | Correspondence between the Knee Identified by TI as Warmer and the Knee Identified by the Clinician with Active Inflammation | |||||
|---|---|---|---|---|---|---|---|---|---|
| Left Knee | Right Knee | Warmer Knee | ATD (°C) | PTD | Knee with Abnormal Warmth | Knee with Mobility Restriction | Knee with Active Inflammation | ||
| 1 | 33.67 | 32.89 | Left | 0.78 | 2.34 | Left | Left | Left | Yes |
| 3 | 30.18 | 31.17 | Right | 0.99 | 3.23 | Right | Right | Right | Yes |
| 5 | 32.17 | 31.97 | Left | 0.20 | 0.62 | Right | Right | Right | No |
| 6 | 30.46 | 30.90 | Right | 0.44 | 1.43 | Right | Right | Right | Yes |
| 9 | 29.61 | 30.60 | Right | 0.99 | 3.29 | Neither | Left | Left | No |
| 10 | 30.20 | 30.45 | Right | 0.25 | 0.82 | Neither | Neither | Right | Yes |
| 11 | 32.61 | 28.78 | Left | 3.83 | 12.48 | Left | Left | Left | Yes |
| 12 | 31.43 | 31.16 | Left | 0.27 | 0.86 | Neither | Neither | Left | Yes |
| Mean Standard deviation | 0.97 1.20 | 3.13 3.92 | |||||||
| Participant | Knee Temperatures Obtained Using Thermal Imaging (°C) | |
|---|---|---|
| Knee with Active Inflammation | Knee without Active Inflammation | |
| 1 | 33.67 | 32.89 |
| 3 | 31.17 | 30.18 |
| 5 | 32.17 | 31.97 |
| 6 | 30.90 | 30.46 |
| 9 | 30.60 | 29.61 |
| 10 | 30.45 | 30.20 |
| 11 | 32.61 | 28.78 |
| 12 | 31.43 | 31.16 |
| Median Minimum Maximum | 31.30 30.45 33.67 | 30.33 28.78 32.89 |
| Mean Standard deviation | 31.63 1.11 | 30.66 1.31 |
| Interquartile range | 1.64 | 1.67 |
| Participant | Mean Temperature by Thermal Imaging (°C) | ATD (°C) | PTD | ||
|---|---|---|---|---|---|
| Left Knee | Right Knee | Common Reference | |||
| 2 | 28.34 | 28.47 | 28.33 | 0.13 | 0.46 |
| 7 | 32.23 | 33.14 | 29.21 | 0.91 | 2.78 |
| 8 | 30.16 | 30.81 | 29.35 | 0.65 | 2.12 |
| 14 | 31.56 | 31.33 | 30.03 | 0.23 | 0.72 |
| 15 | 33.17 | 32.82 | 31.35 | 0.35 | 1.05 |
| 16 | 32.68 | 32.90 | 31.61 | 0.23 | 0.69 |
| 17 | 30.90 | 31.16 | 29.42 | 0.26 | 0.84 |
| 19 | 32.56 | 32.81 | 30.89 | 0.25 | 0.77 |
| Mean Standard deviation | 31.45 1.61 | 31.68 1.59 | 30.02 1.16 | 0.38 0.27 | 1.18 0.82 |
| Median Minimum Maximum | 31.90 28.34 33.17 | 32.07 28.47 33.14 | 29.73 28.33 31.61 | 0.26 0.13 0.91 | 0.81 0.46 2.78 |
| Interquartile range | 2.09 | 1.88 | 1.84 | 0.27 | 0.88 |
| Test | Normality Test Shapiro–Wilk Test; (Confidence Interval, 95%) | Test of Significance in Difference (Paired t-Test; Confidence Interval, 95%) | |||
|---|---|---|---|---|---|
| W | p | Outcome | p | Outcome | |
| Left knee versus reference | 0.958 | 0.872 | Normal | 0.002 | Significant |
| Right knee versus reference | 0.838 | 0.081 | Normal | 0.003 | Significant |
| Left knee versus right knee | 0.947 | 0.762 | Normal | 0.160 | Not significant |
| Participant | Mean Temperature by Thermal Imaging (°C) | ATD (°C) | PTD | |
|---|---|---|---|---|
| Left Knee | Right Knee | |||
| 4 | 31.90 | 31.87 | 0.03 | 0.09 |
| 13 | 30.38 | 30.28 | 0.10 | 0.33 |
| 18 | 32.40 | 32.18 | 0.23 | 0.70 |
| 20 | 31.61 | 31.76 | 0.15 | 0.48 |
| Mean Standard deviation | 31.57 0.86 | 31.52 0.85 | 0.13 0.08 | 0.40 0.26 |
| Measure | Means and Standard Deviations for Groups | Ratios of ATD and PTD | |||
|---|---|---|---|---|---|
| I | II | III | Groups I and III | Group II and III | |
| ATD | 0.97 (1.20) | 0.38 (0.27) | 0.13 (0.08) | 0.97/0.13 = 7.5 | 0.38/0.13 = 2.9 |
| PTD | 3.13 (3.92) | 1.18 (0.82) | 0.40 (0.26) | 3.13/0.40 = 7.8 | 1.18/0.40 = 3.0 |
| Participant | Knee Identified with Active Inflammation by Clinician | Knee Identified Warmer by Thermal Imaging | Knee with Larger Colour Difference against the Common Reference | Correspondence of Colour Analysis and Thermal Imaging | Correspondence of Colour Analysis and Clinical Assessment |
|---|---|---|---|---|---|
| Group I (one knee with active inflammation) | |||||
| 1 | Left | Left | Left | Yes | Yes |
| 3 | Right | Right | Right | Yes | Yes |
| 5 | Right | Left | Left | Yes | No |
| 6 | Right | Right | Left | No | No |
| 9 | Left | Right | Left | No | Yes |
| 10 | Right | Right | Right | Yes | Yes |
| 11 | Left | Left | Right | No | No |
| 12 | Left | Left | Left | Yes | Yes |
| Group II (both knees with active inflammation) | |||||
| 2 | Both | Right | Right | Yes | - |
| 7 | Both | Right | Right | Yes | - |
| 8 | Both | Right | Right | Yes | - |
| 14 | Both | Left | Left | Yes | - |
| 15 | Both | Left | Left | Yes | - |
| 16 | Both | Right | Right | Yes | - |
| 17 | Both | Right | Right | Yes | - |
| 19 | Both | Left | Left | Yes | - |
| Group III (neither knee with active inflammation) | |||||
| 4 | None | Left | Right | No | - |
| 13 | None | Left | Left | Yes | - |
| 18 | None | Left | Left | Yes | - |
| 20 | None | Right | Right | Yes | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nwaizu, H.; Saatchi, R.; Hawley, D.P.; Ward, O. Thermal and Visual Imaging to Assist with Juvenile Idiopathic Arthritis Examination of the Knees. Technologies 2020, 8, 30. https://doi.org/10.3390/technologies8020030
Nwaizu H, Saatchi R, Hawley DP, Ward O. Thermal and Visual Imaging to Assist with Juvenile Idiopathic Arthritis Examination of the Knees. Technologies. 2020; 8(2):30. https://doi.org/10.3390/technologies8020030
Chicago/Turabian StyleNwaizu, Harriet, Reza Saatchi, Daniel Philip Hawley, and Oliver Ward. 2020. "Thermal and Visual Imaging to Assist with Juvenile Idiopathic Arthritis Examination of the Knees" Technologies 8, no. 2: 30. https://doi.org/10.3390/technologies8020030