Phytohormones Regulate Accumulation of Osmolytes Under Abiotic Stress
Abstract
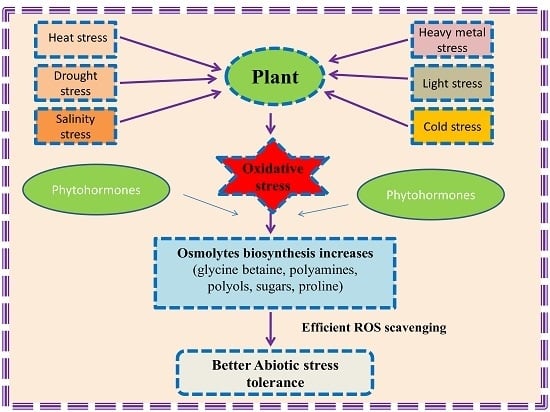
:1. Introduction
2. Involvement of Osmolytes to Bestow Abiotic Stress Tolerance
2.1. Drought Stress
2.2. Salt Stress
2.3. Temperature Stress
2.4. Heavy Metal Stress
2.5. Light Stress
3. Role of Brassinosteroids in Regulation of Osmolytes Under Abiotic Stress
3.1. Brassinosteroids and Proline
3.2. Brassinosteroids and Glycine Betaine
3.3. Brassinosteroids and Polyamines
3.4. Brassinosteroids and Sugars/Sugar Alcohols
4. Role of Ethylene in Regulation of Osmolytes Under Abiotic Stress
4.1. Ethylene and Proline
4.2. Ethylene and Glycine Betaine
4.3. Ethylene and Polyamines
4.4. Ethylene and Sugars/Sugar Alcohols
5. Role of Salicylic Acid in Regulation of Osmolytes Under Abiotic Stress
5.1. Salicylic Acid and Proline
5.2. Salicylic Acid and Glycine Betaine
5.3. Salicylic Acid and Polyamines/other Amino Acids
5.4. Salicylic Acid and Sugar/Sugar Alcohols
6. Role of Cytokinins in Regulation of Osmolytes under Abiotic Stress
6.1. Cytokinins and Proline
6.2. Cytokinins and Glycine Betaine
6.3. Cytokinins and Polyamines
6.4. Cytokinins and Sugars/Sugar Alcohols
7. Role of Jasmonates in Regulation of Osmolytes under Abiotic Stress
7.1. Jasmonates and Proline
7.2. Jasmonates and Glycine Betaine
7.3. Jasmonates and Polyamines
7.4. Jasmonates and Sugars/Sugar Alcohols
8. Role of Abscisic Acid in Regulation of Osmolytes under Abiotic Stress
8.1. Abscisic Acid and Proline
8.2. Abscisic Acid and Glycine Betaine
8.3. Abscisic Acid and Polyamines
8.4. Abscisic Acid and Sugars/Sugar Alcohols
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hussain, S.; Khaliq, A.; Matloob, A.; Wahid, M.A.; Afzal, I. Germination and growth response of three wheat cultivars to NaCl salinity. Soil Environ. 2013, 32, 36–43. [Google Scholar]
- Saud, S.; Chen, Y.; Long, B.; Fahad, S.; Sadiq, A. The different impact on the growth of cool season turf grass under the various conditions on salinity and draught stress. Int. J. Agric. Sci. Res. 2013, 3, 77–84. [Google Scholar]
- Sharma, A.; Yuan, H.; Kumar, V.; Ramakrishnan, M.; Kohli, S.K.; Kaur, R.; Thukral, A.K.; Bhardwaj, R.; Zheng, B. Castasterone attenuates insecticide induced phytotoxicity in mustard. Ecotoxicol. Environ. Saf. 2019, 179, 50–61. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Yuan, H.; Kanwar, M.K.; Bhardwaj, R.; Thukral, A.K.; Zheng, B. Jasmonic Acid Seed Treatment Stimulates Insecticide Detoxification in Brassica juncea L. Front. Plant Sci. 2018, 9, 1609. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Bao, M.; Wang, L.; Khan, I.; Ullah, E.; Tung, S.A.; Samad, R.A.; Shahzad, B. Cadmium toxicity in Maize (Zea mays L.): Consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ. Sci. Pollut. Res. 2015, 22, 17022–17030. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Tanveer, M.; Hassan, W.; Shah, A.N.; Anjum, S.A.; Cheema, S.A.; Ali, I. Lithium toxicity in plants: Reasons, mechanisms and remediation possibilities-A review. Plant Physiol. Biochem. 2016, 107, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; whether toxic or essential for plants and environment-A review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24-Epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2019, 135, 295–303. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Biju, S.; Bhardwaj, R. 24-Epibrassinolide; an active brassinolide and its role in salt stress tolerance in plants: A review. Plant Physiol. Biochem. 2018, 130, 69–79. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Song, H.; Rehman, S.U.; Zhaorong, D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Bray, E.A.; Bailey-Serres, J.W.; Weretilnyk, E. Responses to abiotic stresses. In Biochemistry and Molecular Biology of Plants; Gruissem, W., Jones, R., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; pp. 1158–1203. [Google Scholar]
- Shafi, M.; Bakht, J.; Hassan, M.J.; Raziuddin, M.; Zhang, G. Effect of cadmium and salinity stresses on growth and antioxidant enzyme activities of wheat (Triticum aestivum L.). Bull. Environ. Contam. Toxicol. 2009, 82, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Iqbal, N.; Masood, A.; Khan, N.A. Variation in Salt Tolerance of Wheat Cultivars: Role of Glycinebetaine and Ethylene. Pedosphere 2012, 22, 746–754. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, S.; Kumar, V.; Kanwar, M.K.; Kesavan, A.K.; Thukral, A.K.; Bhardwaj, R.; Alam, P.; Ahmad, P. Pre-sowing Seed Treatment with 24-Epibrassinolide Ameliorates Pesticide Stress in Brassica juncea L. through the Modulation of Stress Markers. Front. Plant Sci. 2016, 7, 1569. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, H.J.; Nelson, D.E.; Jensen, R.G. Adaptations to Environmental Stresses. Plant Cell 1995, 7, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Giri, J. Glycinebetaine and abiotic stress tolerance in plants. Plant Signal. Behav. 2011, 6, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Murata, N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought Induced Changes in Growth, Osmolyte Accumulation and Antioxidant Metabolism of Three Maize Hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Shahzad, B.; Ashraf, U.; Fahad, S.; Hassan, W.; Jan, S.; Khan, I.; Saleem, M.F.; et al. Osmoregulation and antioxidant production in maize under combined cadmium and arsenic stress. Environ. Sci. Pollut. Res. 2016, 23, 11864–11875. [Google Scholar] [CrossRef]
- Taji, T.; Ohsumi, C.; Iuchi, S.; Seki, M.; Kasuga, M.; Kobayashi, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Important roles of drought-and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J. 2002, 29, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Iqbal, N.; Masood, A.; Per, T.S.; Khan, N.A. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal. Behav. 2013, 8, e26374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahzad, B.; Cheema, S.; Farooq, M.; Cheema, Z.; Rehman, A.; Abbas, T. Growth Stimulating Influence of Foliage Applied Brassica Water Extracts on Morphological and Yield Attributes of Bread Wheat under Different Fertilizer Regimes. Planta Daninha 2018, 36. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Chauhan, B.S.; Khan, F.; Ihsan, M.Z.; Ullah, A.; Wu, C.; Bajwa, A.A.; et al. Responses of Rapid Viscoanalyzer Profile and Other Rice Grain Qualities to Exogenously Applied Plant Growth Regulators under High Day and High Night Temperatures. PLoS ONE 2016, 11, e0159590. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Singh, R.; Thukral, A.K.; Bhardwaj, R. Effect of seed pre-soaking with 24-epibrassinolide on growth and photosynthetic parameters of Brassica juncea L. in imidacloprid soil. Ecotoxicol. Environ. Saf. 2016, 133, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Zheng, B. Melatonin Mediated Regulation of Drought Stress: Physiological and Molecular Aspects. Plants 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Soares, C.; Sousa, B.; Martins, M.; Kumar, V.; Shahzad, B.; Sidhu, G.P.S.; Bali, A.S.; Asgher, M.; Bhardwaj, R.; et al. Nitric oxide-mediated regulation of oxidative stress in plants under metal stress: A review on molecular and biochemical aspects. Physiol. Plant. 2019. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Biotechnol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Wani, S.H.; Singh, N.B.; Haribhushan, A.; Mir, J.I. Compatible solute engineering in plants for abiotic stress tolerance-role of glycine betaine. Curr. Genom. 2013, 14, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Alia, P.; Pardha, S.; Prasanna, M. Proline in relation to free radical production in seedlings of Brassica juncea raised under sodium chloride stress. Plant Soil 1993, 155, 497. [Google Scholar] [CrossRef]
- Yancey, P.H. Compatible and counteracting solutes. In Cellular and Molecular Physiology of Cell Volume Regulation; Strange, K., Ed.; CRC Press: Boca Raton, FL, USA, 1994; pp. 81–109. [Google Scholar]
- Ajithkumar, I.P.; Panneerselvam, R. ROS Scavenging System, Osmotic Maintenance, Pigment and Growth Status of Panicum sumatrense Roth. Under Drought Stress. Cell Biochem. Biophys. 2014. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Aref, I.M.; Duarte, A.C.; Pereira, E.; Ahmad, I.; Iqbal, M. Glutathione and proline can coordinately make plants withstand the joint attack of metal (loid) and salinity stresses. Front. Plant Sci. 2017, 5, 662. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.; Meng, Y.-L.; Nii, N. Changes in glycine betaine and related enzyme contents in Amaranthus tricolor under salt stress. J. Plant Physiol. Mol. Biol. 2004, 30, 496–502. [Google Scholar]
- Conde, A.; Silva, P.; Agasse, A.; Conde, C.; Gerós, H. Mannitol transport and mannitol dehydrogenase activities are coordinated in olea europaea under salt and osmotic stresses. Plant Cell Physiol. 2011, 52, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dietz, K.-J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, H.; Chen, T.H.H.; Murata, N. Transformation with a gene for choline oxidase enhances the cold tolerance of Arabidopsis during germination and early growth. Plant Cell Environ. 1998, 21, 232–239. [Google Scholar] [CrossRef]
- Ningthoujam, M.; Habib, K.; Bano, F.; Zutshi, S.; Fatma, T. Exogenous osmolytes suppresses the toxic effects of malathion on Anabaena variabilis. Ecotoxicol. Environ. Saf. 2013, 94, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Gopi, R.; Sankar, B.; Gomathinayagam, M.; Panneerselvam, R. Differential responses in water use efficiency in two varieties of Catharanthus roseus under drought stress. Comptes Rendus Biol. 2008, 331, 42–47. [Google Scholar] [CrossRef]
- Din, J.; Khan, S.; Ali, I.; Gurmani, A. Physiological and agronomic response of canola varieties to drought stress. J. Anim. Plant Sci. 2011, 21, 78–82. [Google Scholar]
- Ashraf, M.; Karim, F. Screening of some cultivars/lines of black gram (Vigna mungo L. Hepper) for resistance to water stress. Trop. Agric. 1991, 68, 57–62. [Google Scholar]
- Serraj, R.; Sinclair, T. Osmolyte accumulation: Can it really help increase crop yield under drought conditions? Plant Cell Environ. 2002, 25, 333–341. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Yamada, M.; Morishita, H.; Urano, K.; Shiozaki, N.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Yoshiba, Y. Effects of free proline accumulation in petunias under drought stress. J. Exp. Bot. 2005, 56, 1975–1981. [Google Scholar] [CrossRef] [Green Version]
- Quan, R.; Shang, M.; Zhang, H.; Zhao, Y.; Zhang, J. Engineering of enhanced glycine betaine synthesis improves drought tolerance in maize. Plant Biotechnol. J. 2004, 2, 477–486. [Google Scholar] [CrossRef]
- Naidu, B.P. Simultaneous estimation of sugars, polyols, proline analogues and betaines accumulating in stressed plants by high performance liquid chromatography-Ultra violet detection. Aust. J. Plant Physiol. 1998, 25, 793–800. [Google Scholar]
- Akashi, K.; Miyake, C.; Yokota, A. Citrulline, a novel compatible solute in drought-tolerant wild watermelon leaves, is an efficient hydroxyl radical scavenger. FEBS Lett. 2001, 508, 438–442. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Qiu, L.; Guo, H.; Wang, Y.; Yuan, H.; Yan, D.; Zheng, B. Spermidine induces physiological and biochemical changes in southern highbush blueberry under drought stress. Braz. J. Bot. 2017, 40, 841–851. [Google Scholar] [CrossRef]
- Cano, E.A.; Pérez-Alfocea, F.; Caro, M.; Bolarín, M.C.; Moreno, V. Evaluation of salt tolerance in cultivated and wild tomato species through in vitro shoot apex culture. Plant Cell Tissue Organ Cult. 1998, 53, 19–26. [Google Scholar] [CrossRef]
- Khan, M.I.; Asgher, M.; Khan, N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol. Biochem. 2014, 80, 67–74. [Google Scholar] [CrossRef]
- Nazar, R.; Khan, M.I.; Iqbal, N.; Masood, A.; Khan, N.A. Involvement of ethylene in reversal of salt-inhibited photosynthesis by sulfur in mustard. Physiol. Plant. 2014, 152, 331–344. [Google Scholar] [CrossRef]
- Tomescu, D.; Şumălan, R.; Copolovici, L.; Copolovici, D. The influence of soil salinity on volatile organic compounds emission and photosynthetic parameters of Solanum lycopersicum L. varieties. Open Life Sci. 2017, 12, 135–142. [Google Scholar] [CrossRef]
- Tang, W.; Luo, C. Overexpression of Zinc Finger Transcription Factor ZAT6 Enhances Salt Tolerance. Open Life Sci. 2018, 13, 431–445. [Google Scholar] [CrossRef]
- Zhu, J.-K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Sudhakar, C.; Lakshmi, A.; Giridarakumar, S. Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci. 2001, 161, 613–619. [Google Scholar] [CrossRef]
- Sumithra, K.; Jutur, P.; Carmel, B.D.; Reddy, A.R. Salinity-induced changes in two cultivars of Vigna radiata: Responses of antioxidative and proline metabolism. Plant Growth Regul. 2006, 50, 11–22. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Plant Response to Salt Stress and Role of Exogenous Protectants to Mitigate Salt-Induced Damages. In Ecophysiology and Responses of Plants under Salt Stress; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2013; pp. 25–87. [Google Scholar]
- Khedr, A.H.; Abbas, M.A.; Wahid, A.A.; Quick, W.P.; Abogadallah, G.M. Proline induces the expression of salt-stress-responsive proteins and may improve the adaptation of Pancratium maritimum L. to salt-stress. J. Exp. Bot. 2003, 54, 2553–2562. [Google Scholar] [CrossRef]
- Hoque, M.A.; Banu, M.N.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and glycinebetaine enhance antioxidant defense and methylglyoxal detoxification systems and reduce NaCl-induced damage in cultured tobacco cells. J. Plant Physiol. 2008, 165, 813–824. [Google Scholar] [CrossRef]
- Chen, T.H.; Murata, N. Glycinebetaine: An effective protectant against abiotic stress in plants. Trends Plant Sci. 2008, 13, 499–505. [Google Scholar] [CrossRef]
- Mäkelä, P.; Kärkkäinen, J.; Somersalo, S. Effect of Glycinebetaine on Chloroplast Ultrastructure, Chlorophyll and Protein Content, and RuBPCO Activities in Tomato Grown under Drought or Salinity. Biol. Plant. 2000, 43, 471–475. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Lutts, S. Exogenous glycinebetaine reduces sodium accumulation in salt-stressed rice plants. Int. Rice Res. Notes 2000, 25, 39–40. [Google Scholar]
- Reda, F.; Mandoura, H.M.H. Response of enzymes activities, photosynthetic pigments, proline to low or high temperature stressed wheat plant (Triticum aestivum L.) in the presence or absence of exogenous proline or cysteine. Int. J. Acad. Res. 2011, 3, 108–115. [Google Scholar]
- Li, J.; Pandeya, D.; Nath, K.; Zulfugarov, I.S.; Yoo, S.C.; Zhang, H.; Yoo, J.H.; Cho, S.H.; Koh, H.J.; Kim, D.S. ZEBRA-NECROSIS, a thylakoid-bound protein, is critical for the photoprotection of developing chloroplasts during early leaf development. Plant J. 2010, 62, 713–725. [Google Scholar] [CrossRef]
- Tewari, A.K.; Tripathy, B.C. Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat. Plant Physiol. 1998, 117, 851–858. [Google Scholar] [CrossRef]
- Xu, Z.-S.; Xia, L.-Q.; Chen, M.; Cheng, X.-G.; Zhang, R.-Y.; Li, L.-C.; Zhao, Y.-X.; Lu, Y.; Ni, Z.-Y.; Liu, L. Isolation and molecular characterization of the Triticum aestivum L. ethylene-responsive factor 1 (TaERF1) that increases multiple stress tolerance. Plant Mol. Biol. 2007, 65, 719–732. [Google Scholar] [CrossRef]
- Kishor, P.K.; Sangam, S.; Amrutha, R.; Laxmi, P.S.; Naidu, K.; Rao, K.; Rao, S.; Reddy, K.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Vaida, J.; Natalija, B.; Ramune, K.; Ausra, B. Effect of exogenous proline and de-acclimation treatment on cold tolerance in Brassica napus shoots cultured in vitro. J. Food Agric. Environ. 2012, 10, 327–330. [Google Scholar]
- Kawakami, A.; Sato, Y.; Yoshida, M. Genetic engineering of rice capable of synthesizing fructans and enhancing chilling tolerance. J. Exp. Bot. 2008, 59, 793–802. [Google Scholar] [CrossRef] [Green Version]
- Kathuria, H.; Giri, J.; Nataraja, K.N.; Murata, N.; Udayakumar, M.; Tyagi, A.K. Glycinebetaine-induced water-stress tolerance in codA-expressing transgenic indica rice is associated with up-regulation of several stress responsive genes. Plant Biotechnol. J. 2009, 7, 512–526. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Combined effect of 24-epibrassinolide and salicylic acid mitigates lead (Pb) toxicity by modulating various metabolites in Brassica juncea L. seedlings. Protoplasma 2018, 255, 11–24. [Google Scholar] [CrossRef]
- Sharma, J.; Chakraverty, N. Mechanism of plant tolerance in response to heavy metals. In Molecular Stress Physiology of Plants; Das, A.B., Rout, G.R., Eds.; Springer: Berlin, Germany, 2013; pp. 289–308. [Google Scholar]
- Fahad, S.; Rehman, A.; Shahzad, B.; Tanveer, M.; Saud, S.; Kamran, M.; Ihtisham, M.; Khan, S.U.; Turan, V.; ur Rahman, M.H. Rice Responses and Tolerance to Metal/Metalloid Toxicity. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J.K., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 299–312. [Google Scholar]
- Guo, H.; Chen, H.; Hong, C.; Jiang, D.; Zheng, B. Exogenous malic acid alleviates cadmium toxicity in Miscanthus sacchariflorus through enhancing photosynthetic capacity and restraining ROS accumulation. Ecotoxicol. Environ. Saf. 2017, 141, 119–128. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Dugardeyn, J.; Van Der Straeten, D. Ethylene: Fine-tuning plant growth and development by stimulation and inhibition of elongation. Plant Sci. 2008, 175, 59–70. [Google Scholar] [CrossRef]
- Guo, H.; Feng, X.; Hong, C.; Chen, H.; Zeng, F.; Zheng, B.; Jiang, D. Malate secretion from the root system is an important reason for higher resistance of Miscanthus sacchariflorus to cadmium. Physiol. Plant. 2017, 159, 340–353. [Google Scholar] [CrossRef]
- Sidhu, G.P.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Appraising the role of environment friendly chelants in alleviating lead by Coronopus didymus from Pb-contaminated soils. Chemosphere 2017, 182, 129–136. [Google Scholar] [CrossRef]
- Malik, A. Metal bioremediation through growing cells. Environ. Int. 2004, 30, 261–278. [Google Scholar] [CrossRef]
- Dhir, B.; Nasim, S.A.; Samantary, S.; Srivastava, S. Assessment of Osmolyte Accumulation in Heavy Metal Exposed Salvinia natans. Int. J. Bot. 2012, 8, 153–158. [Google Scholar] [Green Version]
- Bhatti, K.H.; Anwar, S.; Nawaz, K.; Hussain, K.; Siddiqi, E.; Sharif, R.; Talat, A.; Khalid, A. Effect of exogenous application of glycinebetaine on wheat (Triticum aestivum L.) under heavy metal stress. Middle East J. Sci. Res. 2013, 14, 130–137. [Google Scholar]
- Kulheim, C.; Agren, J.; Jansson, S. Rapid regulation of light harvesting and plant fitness in the field. Science 2002, 297, 91–93. [Google Scholar] [CrossRef]
- Kirchhoff, H. Structural changes of the thylakoid membrane network induced by high light stress in plant chloroplasts. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130225. [Google Scholar] [CrossRef] [Green Version]
- Miyazawa, S.; Livingston, N.J.; Turpin, D.H. Stomatal development in new leaves is related to the stomatal conductance of mature leaves in poplar (Populus trichocarpa x P. deltoides). J. Exp. Bot. 2006, 57, 373–380. [Google Scholar] [CrossRef]
- Hideg, E.; Jansen, M.A.; Strid, A. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Hou, P.; Su, X.; Zhao, P.; Zhao, H.; Liu, S. Foliar-applied salicylic acid alleviates heat and high light stress induced photoinhibition in wheat (Triticum aestivum) during the grain filling stage by modulating the psbA gene transcription and antioxidant defense. Plant Growth Regul. 2014, 73, 289–297. [Google Scholar] [CrossRef]
- Fujioka, S.; Yokota, T. Biosynthesis and Metabolism of Brassinosteroids. Annu. Rev. Plant Biol. 2003, 54, 137–164. [Google Scholar] [CrossRef]
- Vardhini, B.V.; Anuradha, S.; Sujatha, E.; Rao, S.S.R. Role of brassinosteroids in alleviating various abiotic and biotic stresses-a review. Plant Nutr. Abiotic Stress Toler. I Plant Stress 2010, 4, 56–61. [Google Scholar]
- Choudhary, S.P.; Oral, H.V.; Bhardwaj, R.; Yu, J.-Q.; Tran, L.-S.P. Interaction of brassinosteroids and polyamines enhances copper stress tolerance in Raphanus sativus. J. Exp. Bot. 2012, 63, 5659–5675. [Google Scholar] [CrossRef]
- Rattan, A.; Kapoor, D.; Kapoor, N.; Bhardwaj, R. Application of brassionsteroids reverses the inhibitory effect of salt stress on growth and photosynthetic activity of Zea mays plants. Int. J. Theor. Appl. Sci. 2014, 6, 13–22. [Google Scholar]
- Coll, Y.; Coll, F.; Amoros, A.; Pujol, M. Brassinosteroids roles and applications: An up-date. Biologia 2015, 70, 726–732. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Bali, S.; Arora, S.; Sharma, A.; Kaur, R.; Bhardwaj, R. Modulation of antioxidative defense expression and osmolyte content by co-application of 24-epibrassinolide and salicylic acid in Pb exposed Indian mustard plants. Ecotoxicol. Environ. Saf. 2018, 147, 382–393. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Interaction of 24-epibrassinolide and salicylic acid regulates pigment contents, antioxidative defense responses, and gene expression in Brassica juncea L. seedlings under Pb stress. Environ. Sci. Pollut. Res. 2018, 25, 15159–15173. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Kumar, R.; Shahzad, B.; Thukral, A.K.; Bhardwaj, R. Brassinosteroid-mediated pesticide detoxification in plants: A mini-review. Cogent Food Agric. 2018, 4, 1436212. [Google Scholar] [CrossRef]
- Rehman, S.; Shahzad, B.; Bajwa, A.A.; Hussain, S.; Rehman, A.; Cheema, S.A.; Abbas, T.; Ali, A.; Shah, L.; Adkins, S.; et al. Utilizing the Allelopathic Potential of Brassica Species for Sustainable Crop Production: A Review. J. Plant Growth Regul. 2019, 38, 343–356. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Kanwar, M.K.; Thukral, A.K.; Bhardwaj, R. Ameliorating imidacloprid induced oxidative stress by 24-epibrassinolide in Brassica juncea L. Russ. J. Plant Physiol. 2017, 64, 509–517. [Google Scholar] [CrossRef]
- Handa, N.; Kohli, S.K.; Kaur, R.; Sharma, A.; Kumar, V.; Thukral, A.K.; Arora, S.; Bhardwaj, R. Role of Compatible Solutes in Enhancing Antioxidative Defense in Plants Exposed to Metal Toxicity. In Plants under Metal and Metalloid Stress: Responses, Tolerance and Remediation; Hasanuzzaman, M., Nahar, K., Fujita, M., Eds.; Springer: Singapore, 2018; pp. 207–228. [Google Scholar]
- Kaur, R.; Yadav, P.; Sharma, A.; Kumar Thukral, A.; Kumar, V.; Kaur Kohli, S.; Bhardwaj, R. Castasterone and citric acid treatment restores photosynthetic attributes in Brassica juncea L. under Cd(II) toxicity. Ecotoxicol. Environ. Saf. 2017, 145, 466–475. [Google Scholar] [CrossRef]
- Fàbregas, N.; Lozano-Elena, F.; Blasco-Escámez, D.; Tohge, T.; Martínez-Andújar, C.; Albacete, A.; Osorio, S.; Bustamante, M.; Riechmann, J.L.; Nomura, T.; et al. Overexpression of the vascular brassinosteroid receptor BRL3 confers drought resistance without penalizing plant growth. Nat. Commun. 2018, 9, 4680. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Kido, E.A.; Neto, J.R.F.; Silva, R.L.; Belarmino, L.C.; Neto, J.P.B.; Soares-Cavalcanti, N.M.; Pandolfi, V.; Silva, M.D.; Nepomuceno, A.L.; Benko-Iseppon, A.M. Expression dynamics and genome distribution of osmoprotectants in soybean: Identifying important components to face abiotic stress. BMC Bioinform. 2013, 14, S7. [Google Scholar] [CrossRef]
- Kumar, K.; Kumar, M.; Kim, S.-R.; Ryu, H.; Cho, Y.-G. Insights into genomics of salt stress response in rice. Rice 2013, 6, 27. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Bhardwaj, R.; Gupta, B.D.; Dutt, P.; Gupta, R.K.; Biondi, S.; Kanwar, M. Epibrassinolide induces changes in indole-3-acetic acid, abscisic acid and polyamine concentrations and enhances antioxidant potential of radish seedlings under copper stress. Physiol. Plant. 2010, 140, 280–296. [Google Scholar] [CrossRef]
- Sharma, I.; Pati, P.K.; Bhardwaj, R. Effect of 24-epibrassinolide on oxidative stress markers induced by nickel-ion in Raphanus sativus L. Acta Physiol. Plant. 2011, 33, 1723–1735. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Khalil, R.R.; Mir, B.A.; Yusuf, M.; Ahmad, A. 24-Epibrassinolide regulates photosynthesis, antioxidant enzyme activities and proline content of Cucumis sativus under salt and/or copper stress. Environ. Monit. Assess. 2013, 185, 7845–7856. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, P.; Jiang, Y.; Wang, M.; Zhang, L. Effects of brassinosteroid on antioxidant system in Salvia miltiorrhiza under drought stress. J. Res. Agric. Anim. Sci. 2014, 2, 1–6. [Google Scholar]
- Khamsuk, O.; Sonjaroon, W.; Suwanwong, S.; Jutamanee, K.; Suksamrarn, A. Effects of 24-epibrassinolide and the synthetic brassinosteroid mimic on chili pepper under drought. Acta Physiol. Plant. 2018, 40, 106. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Yusuf, M.; Hayat, S.; Ahmad, A. Effect of 28-homobrassinolide on antioxidant capacity and photosynthesis in Brassica juncea plants exposed to different levels of copper. Environ. Exp. Bot. 2009, 66, 418–424. [Google Scholar] [CrossRef]
- Lalotra, S.; Hemantaranjan, A.; Kumar, S.; Kant, R. Effect of brassinosteroid (brassinolide) on seedling traits, morphology and metabolism in mung bean under salinity stress. Annu. Res. Rev. Biol. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.; Lv, X.; Cheng, N.; Peng, B.; Cao, W. Effect of 24-epibrassinolide on chilling injury of peach fruit in relation to phenolic and proline metabolisms. Postharvest Biol. Technol. 2016, 111, 390–397. [Google Scholar] [CrossRef]
- Hare, P.; Cress, W.; Van Staden, J. Proline synthesis and degradation: A model system for elucidating stress-related signal transduction. J. Exp. Bot. 1999, 50, 413–434. [Google Scholar] [CrossRef]
- Delauney, A.J.; Verma, D.P.S. Proline biosynthesis and osmoregulation in plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Wani, A.S.; Ahmad, A.; Hayat, S.; Tahir, I. Epibrassinolide and proline alleviate the photosynthetic and yield inhibition under salt stress by acting on antioxidant system in mustard. Plant Physiol. Biochem. 2019, 135, 385–394. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Ozga, J.A.; Zaman, M.; Pharis, R.P. The physiology of plant hormones in cereal, oilseed and pulse crops. Prairie Soils Crop. 2013, 6, 7–23. [Google Scholar]
- Kurepin, L.V.; Ivanov, A.G.; Zaman, M.; Pharis, R.P.; Allakhverdiev, S.I.; Hurry, V.; Huner, N.P. Stress-related hormones and glycinebetaine interplay in protection of photosynthesis under abiotic stress conditions. Photosynth. Res. 2015, 126, 221–235. [Google Scholar] [CrossRef]
- Huang, J.; Hirji, R.; Adam, L.; Rozwadowski, K.L.; Hammerlindl, J.K.; Keller, W.A.; Selvaraj, G. Genetic engineering of glycinebetaine production toward enhancing stress tolerance in plants: metabolic limitations. Plant Physiol. 2000, 122, 747–756. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Ivanov, A.G.; Zaman, M.; Pharis, R.P.; Hurry, V.; Hüner, N.P. Interaction of glycine betaine and plant hormones: Protection of the photosynthetic apparatus during abiotic stress. In Photosynthesis: Structures, Mechanisms, and Applications; Hou, H.J.M., Najafpour, M.M., Moore, G.F., Allakhverdiev, S.I., Eds.; Springer: Berlin, Germany, 2017; pp. 185–202. [Google Scholar]
- Park, E.J.; Jeknić, Z.; Pino, M.T.; Murata, N.; Chen, T.H.H. Glycinebetaine accumulation is more effective in chloroplasts than in the cytosol for protecting transgenic tomato plants against abiotic stress. Plant Cell Environ. 2007, 30, 994–1005. [Google Scholar] [CrossRef]
- Mäkelä, P.; Peltonen-Sainio, P.; Jokinen, K.; Pehu, E.; Setälä, H.; Hinkkanen, R.; Somersalo, S. Uptake and translocation of foliar-applied glycinebetaine in crop plants. Plant Sci. 1996, 121, 221–230. [Google Scholar] [CrossRef]
- Hattori, T.; Mitsuya, S.; Fujiwara, T.; Jagendorf, A.T.; Takabe, T. Tissue specificity of glycinebetaine synthesis in barley. Plant Sci. 2009, 176, 112–118. [Google Scholar] [CrossRef]
- Adams, W.W.; Muller, O.; Cohu, C.M.; Demmig-Adams, B. May photoinhibition be a consequence, rather than a cause, of limited plant productivity? Photosynth. Res. 2013, 117, 31–44. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Kanwar, M.; Bhardwaj, R.; Gupta, B.; Gupta, R. Epibrassinolide ameliorates Cr (VI) stress via influencing the levels of indole-3-acetic acid, abscisic acid, polyamines and antioxidant system of radish seedlings. Chemosphere 2011, 84, 592–600. [Google Scholar] [CrossRef]
- Shahid, M.A.; Balal, R.M.; Pervez, M.A.; Garcia-Sanchez, F.; Gimeno, V.; Abbas, T.; Mattson, N.S.; Riaz, A. Treatment with 24-epibrassinolide mitigates NaCl-induced toxicity by enhancing carbohydrate metabolism, osmolyte accumulation, and antioxidant activity in Pisum sativum. Turk. J. Bot. 2014, 38, 511–525. [Google Scholar] [CrossRef]
- Ahmad, P.; Abd_Allah, E.F.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Siddique, K.H. Exogenous application of calcium to 24-epibrassinosteroid pre-treated tomato seedlings mitigates NaCl toxicity by modifying ascorbate–glutathione cycle and secondary metabolites. Sci. Rep. 2018, 8, 13515. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Siddique, K.H.; Ahmad, P. Interactive effect of 24-epibrassinolide and silicon alleviates cadmium stress via the modulation of antioxidant defense and glyoxalase systems and macronutrient content in Pisum sativum L. seedlings. BMC Plant Biol. 2018, 18, 146. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, B.; Shahbaz, M.; Akram, N.A. Interactive effect of foliar application of 24-epibrassinolide and root zone salinity on morpho-physiological attributes of wheat (Triticum aestivum L.). Int. J. Agric. Biol. 2007, 9, 584–589. [Google Scholar]
- Sengupta, A.; Chakraborty, M.; Saha, J.; Gupta, B.; Gupta, K. Polyamines: Osmoprotectants in plant abiotic stress adaptation. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Iqbal, N., Nazar, R., Khan, N.A., Eds.; Springer: Berlin, Germany, 2016; pp. 97–127. [Google Scholar]
- Liu, J.-H.; Wang, W.; Wu, H.; Gong, X.; Moriguchi, T. Polyamines function in stress tolerance: From synthesis to regulation. Front. Plant Sci. 2015, 6, 827. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, J.; Liu, K.; Wang, Z.; Liu, L. Involvement of polyamines in the drought resistance of rice. J. Exp. Bot. 2007, 58, 1545–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, T.; Kakehi, J.-I. Polyamines: Ubiquitous polycations with unique roles in growth and stress responses. Ann. Bot. 2009, 105, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, V.V.; Shevyakova, N.I. Polyamines and Plant Adaptation to Saline Environments. In Desert Plants: Biology and Biotechnology; Ramawat, K.G., Ed.; Springer: Berlin, Germany, 2010; pp. 261–298. [Google Scholar]
- Parvaiz, A.; Satyawati, S. Salt stress and phyto-biochemical responses of plants-a review. Plant Soil Environ. 2008, 54, 89. [Google Scholar] [CrossRef]
- Murakeozy, E.P.; Nagy, Z.; Duhaze, C.; Bouchereau, A.; Tuba, Z. Seasonal changes in the levels of compatible osmolytes in three halophytic species of inland saline vegetation in Hungary. J. Plant Physiol. 2003, 160, 395–401. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J. Sugar-induced plant growth is dependent on brassinosteroids. Plant Signal. Behav. 2015, 10, e1082700. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, Z.; Wang, J.; Chen, Y.; Bi, Y.; He, J. Brassinosteroid is required for sugar promotion of hypocotyl elongation in Arabidopsis in darkness. Planta 2015, 242, 881–893. [Google Scholar] [CrossRef]
- Osborne, D.J. Ethylene in Plant Biology, 2nd ed.; Oxford University Press: Oxford, UK, 1993; Volume 44, p. 687. [Google Scholar]
- Cao, W.-H.; Liu, J.; He, X.-J.; Mu, R.-L.; Zhou, H.-L.; Chen, S.-Y.; Zhang, J.-S. Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol. 2007, 143, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Etheridge, N.; Schaller, G.E. Ethylene signal transduction. Ann. Bot. 2005, 95, 901–915. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, J.S. Ethylene biosynthesis and signaling pathway. Chin. Bull. Bot. 2006, 5, 519–530. [Google Scholar]
- Hattori, Y.; Nagai, K.; Furukawa, S.; Song, X.-J.; Kawano, R.; Sakakibara, H.; Wu, J.; Matsumoto, T.; Yoshimura, A.; Kitano, H.; et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 2009, 460, 1026. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Umar, S.; Khan, N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 2015, 178, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Lin, Y.; Zu, Y.; Efferth, T.; Li, D.; Tang, Z. Ethylene increases accumulation of compatible solutes and decreases oxidative stress to improve plant tolerance to water stress in Arabidopsis. J. Plant Biol. 2015, 58, 193–201. [Google Scholar] [CrossRef]
- Filippou, P.; Bouchagier, P.; Skotti, E.; Fotopoulos, V. Proline and reactive oxygen/nitrogen species metabolism is involved in the tolerant response of the invasive plant species Ailanthus altissima to drought and salinity. Environ. Exp. Bot. 2014, 97, 1–10. [Google Scholar] [CrossRef]
- Nounjan, N.; Nghia, P.T.; Theerakulpisut, P. Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J. Plant Physiol. 2012, 169, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Mushtaq, Z.; Andaz, F.; Masood, A. Does proline application ameliorate adverse effects of salt stress on growth, ions and photosynthetic ability of eggplant (Solanum melongena L.)? Sci. Hortic. 2013, 164, 507–511. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Khan, M.I.R. A new perspective of phytohormones in salinity tolerance: Regulation of proline metabolism. Environ. Exp. Bot. 2014, 100, 34–42. [Google Scholar] [CrossRef]
- Khan, M.I.; Nazir, F.; Asgher, M.; Per, T.S.; Khan, N.A. Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J. Plant Physiol. 2015, 173, 9–18. [Google Scholar] [CrossRef]
- Chrominski, A.; Halls, S.; Weber, D.; Smith, B. Proline affects ACC to ethylene conversion under salt and water stresses in the halophyte, Allenrolfea occidentalis. Environ. Exp. Bot. 1989, 29, 359–363. [Google Scholar] [CrossRef]
- Ranganayakulu, G.S.; Veeranagamallaiah, G.; Sudhakar, C. Effect of salt stress on osmolyte accumulation in two groundnut cultivars (Arachis hypogaea L.) with contrasting salt tolerance. Afr. J. Plant Sci. 2013, 7, 586–592. [Google Scholar]
- Walton, L.J.; Kurepin, L.V.; Yeung, E.C.; Shah, S.; Emery, R.N.; Reid, D.M.; Pharis, R.P. Ethylene involvement in silique and seed development of canola, Brassica napus L. Plant Physiol. Biochem. 2012, 58, 142–150. [Google Scholar] [CrossRef]
- Morgan, P.W.; Drew, M.C. Ethylene and plant responses to stress. Physiol. Plant. 1997, 100, 620–630. [Google Scholar] [CrossRef]
- Park, E.J.; Jeknic, Z.; Sakamoto, A.; DeNoma, J.; Yuwansiri, R.; Murata, N.; Chen, T.H. Genetic engineering of glycinebetaine synthesis in tomato protects seeds, plants, and flowers from chilling damage. Plant J. 2004, 40, 474–487. [Google Scholar] [CrossRef]
- Wang, G.; Li, F.; Zhang, J.; Zhao, M.; Hui, Z.; Wang, W. Overaccumulation of glycine betaine enhances tolerance of the photosynthetic apparatus to drought and heat stress in wheat. Photosynthetica 2010, 48, 30–41. [Google Scholar] [CrossRef]
- Allard, F.; Houde, M.; Sarhan, F.; Kröl, M.; Ivanov, A.; Huner, N.P.A. Betaine Improves Freezing Tolerance in Wheat. Plant Cell Physiol. 1998, 39, 1194–1202. [Google Scholar] [CrossRef]
- Quan, R.; Shang, M.; Zhang, H.; Zhao, Y.; Zhang, J. Improved chilling tolerance by transformation with betA gene for the enhancement of glycinebetaine synthesis in maize. Plant Sci. 2004, 166, 141–149. [Google Scholar] [CrossRef]
- Hussain, S.S.; Ali, M.; Ahmad, M.; Siddique, K.H. Polyamines: Natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol. Adv. 2011, 29, 300–311. [Google Scholar] [CrossRef]
- Kubiś, J.; Floryszak-Wieczorek, J.; Arasimowicz-Jelonek, M. Polyamines induce adaptive responses in water deficit stressed cucumber roots. J. Plant Res. 2014, 127, 151–158. [Google Scholar] [CrossRef]
- Petruzzelli, L.; Coraggio, I.; Leubner-Metzger, G. Ethylene promotes ethylene biosynthesis during pea seed germination by positive feedback regulation of 1-aminocyclo-propane-1-carboxylic acid oxidase. Planta 2000, 211, 144–149. [Google Scholar] [CrossRef]
- Li, C.-Z.; Jiao, J.; Wang, G.-X. The important roles of reactive oxygen species in the relationship between ethylene and polyamines in leaves of spring wheat seedlings under root osmotic stress. Plant Sci. 2004, 166, 303–315. [Google Scholar] [CrossRef]
- Li, C.-z.; Wang, G.-x. Interactions between reactive oxygen species, ethylene and polyamines in leaves of Glycyrrhiza inflata seedlings under root osmotic stress. Plant Growth Regul. 2004, 42, 55–60. [Google Scholar] [CrossRef]
- Van den Ende, W.; Valluru, R. Sucrose, sucrosyl oligosaccharides, and oxidative stress: Scavenging and salvaging? J. Exp. Bot. 2008, 60, 9–18. [Google Scholar] [CrossRef]
- Koyro, H.-W.; Ahmad, P.; Geissler, N. Abiotic Stress Responses in Plants: An Overview. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 1–28. [Google Scholar]
- Kerepesi, I.; Galiba, G. Osmotic and Salt Stress-Induced Alteration in Soluble Carbohydrate Content in Wheat Seedlings. Crop Sci. 2000, 40, 482–487. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; Terry, N.; Sears, T.; van Dun, K. Enhanced drought resistance in fructan-producing sugar beet. Plant Physiol. Biochem. 1999, 37, 313–317. [Google Scholar] [CrossRef]
- Luo, Y.; Li, F.; Wang, G.; Yang, X.; Wang, W. Exogenously-supplied trehalose protects thylakoid membranes of winter wheat from heat-induced damage. Biol. Plant. 2010, 54, 495–501. [Google Scholar] [CrossRef]
- Theerakulpisut, P.; Gunnula, W. Exogenous sorbitol and trehalose mitigated salt stress damage in salt-sensitive but not salt-tolerant rice seedlings. Asian J. Crop Sci. 2012, 4, 165–170. [Google Scholar] [CrossRef]
- Li, H.W.; Zang, B.S.; Deng, X.W.; Wang, X.P. Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice. Planta 2011, 234, 1007–1018. [Google Scholar] [CrossRef]
- Kanayama, Y.; Watanabe, M.; Moriguchi, R.; Deguchi, M.; Kanahama, K.; Yamaki, S. Effects of Low Temperature and Abscisic Acid on the Expression of the Sorbitol-6-phosphate Dehydrogenase Gene in Apple Leaves. J. Jpn. Soc. Hortic. Sci. 2006, 75, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Patonnier, M.P.; Peltier, J.P.; Marigo, G. Drought-induced increase in xylem malate and mannitol concentrations and closure of Fraxinus excelsior L. stomata. J. Exp. Bot. 1999, 50, 1223–1229. [Google Scholar] [CrossRef]
- Kaya, C.; Sonmez, O.; Aydemir, S.; Ashraf, M.; Dikilitas, M. Exogenous application of mannitol and thiourea regulates plant growth and oxidative stress responses in salt-stressed maize (Zea mays L.). J. Plant Interact. 2013, 8, 234–241. [Google Scholar] [CrossRef]
- Ahn, C.; Park, U.; Park, P.B. Increased salt and drought tolerance by d-ononitol production in transgenic Arabidopsis thaliana. Biochem. Biophy. Res. Commun. 2011, 415, 669–674. [Google Scholar] [CrossRef]
- Misra, N.; Saxena, P. Effect of salicylic acid on proline metabolism in lentil grown under salinity stress. Plant Sci. 2009, 177, 181–189. [Google Scholar] [CrossRef]
- Misra, N.; Misra, R. Salicylic acid changes plant growth parameters and proline metabolism in Rauwolfia serpentina leaves grown under salinity stress. Am. Eurasian J. Agric. Environ. Sci. 2012, 12, 1601–1609. [Google Scholar]
- Zengin, F. Exogenous treatment with salicylic acid alleviating copper toxicity in bean seedlings. Proceed. Nat. Acad. Sci. Ind. Sec. B Biol. Sci. 2014, 84, 749–755. [Google Scholar] [CrossRef]
- Yuan, Z.; Cong, G.; Zhang, J. Effects of exogenous salicylic acid on polysaccharides production of Dendrobium officinale. S. Afr. J. Bot. 2014, 95, 78–84. [Google Scholar] [CrossRef]
- Chen, T.H.; Murata, N. Glycinebetaine protects plants against abiotic stress: Mechanisms and biotechnological applications. Plant Cell Environ. 2011, 34, 1–20. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.; Du, X.; Tang, H.; Shen, C.; Wu, J. Salicylic acid alleviates the adverse effects of salt stress in Torreya grandis cv. Merrillii seedlings by activating photosynthesis and enhancing antioxidant systems. PLoS ONE 2014, 9, e109492. [Google Scholar] [CrossRef]
- Hayat, S.; Masood, A.; Yusuf, M.; Fariduddin, Q.; Ahmad, A. Growth of Indian mustard (Brassica juncea L.) in response to salicylic acid under high-temperature stress. Braz. J. Plant Physiol. 2009, 21, 187–195. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, J.; Zhang, X.; Hu, Q.; Qian, R. Salicylic acid alleviates the adverse effects of salt stress on Dianthus superbus (Caryophyllaceae) by activating photosynthesis, protecting morphological structure, and enhancing the antioxidant system. Front. Plant Sci. 2017, 8, 600. [Google Scholar] [CrossRef]
- La, V.H.; Lee, B.-R.; Zhang, Q.; Park, S.-H.; Islam, M.T.; Kim, T.-H. Salicylic acid improves drought-stress tolerance by regulating the redox status and proline metabolism in Brassica rapa. Hortic. Environ. Biotechnol. 2019, 60, 31–40. [Google Scholar] [CrossRef]
- Jagendorf, A.T.; Takabe, T. Inducers of glycinebetaine synthesis in barley. Plant Physiol. 2001, 127, 1827–1835. [Google Scholar] [CrossRef]
- Hoyos, M.E.; Zhang, S. Calcium-independent activation of salicylic acid-induced protein kinase and a 40-kilodalton protein kinase by hyperosmotic stress. Plant Physiol. 2000, 122, 1355–1364. [Google Scholar] [CrossRef]
- Aldesuquy, H.S.; Abbas, M.A.; Abo-Hamed, S.A.; Elhakem, A.H.; Alsokari, S.S. Glycine betaine and salicylic acid induced modification in productivity of two different cultivars of wheat grown under water stress. J. Stress Physiol. Biochem. 2012, 8, 72–89. [Google Scholar]
- Hussain, M.; Malik, M.; Farooq, M.; Khan, M.; Akram, M.; Saleem, M. Exogenous glycinebetaine and salicylic acid application improves water relations, allometry and quality of hybrid sunflower under water deficit conditions. J. Agron. Crop Sci. 2009, 195, 98–109. [Google Scholar] [CrossRef]
- Gharbi, E.; Martínez, J.-P.; Benahmed, H.; Fauconnier, M.-L.; Lutts, S.; Quinet, M. Salicylic acid differently impacts ethylene and polyamine synthesis in the glycophyte Solanum lycopersicum and the wild-related halophyte Solanum chilense exposed to mild salt stress. Physiol. Plant. 2016, 158, 152–167. [Google Scholar] [CrossRef]
- Liu, G.; Ji, Y.; Bhuiyan, N.H.; Pilot, G.; Selvaraj, G.; Zou, J.; Wei, Y. Amino acid homeostasis modulates salicylic acid–associated redox status and defense responses in Arabidopsis. Plant Cell 2010, 22, 3845–3863. [Google Scholar] [CrossRef]
- van Damme, M.; Zeilmaker, T.; Elberse, J.; Andel, A.; de Sain-van der Velden, M.; van den Ackerveken, G. Downy mildew resistance in Arabidopsis by mutation of HOMOSERINE KINASE. Plant Cell 2009, 21, 2179–2189. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. Improving amino acid composition of soybean under salt stress by salicylic acid and jasmonic acid. J. Appl. Bot. Food Qual. 2016, 89, 243–248. [Google Scholar]
- Zushi, K. Comparison of chemical composition contents of tomato fruit grown under water and salinity stresses. J. SHITA 2005, 17, 128–136. [Google Scholar] [CrossRef]
- Yadav, S.; Lakshmi, N.J.; Maheswari, M.; Vanaja, M.; Venkateswarlu, B. Influence of water deficit at vegetative, anthesis and grain filling stages on water relation and grain yield in sorghum. Indian J. Plant Physiol. 2005, 10, 20–24. [Google Scholar]
- Sankar, B.; Jaleel, C.A.; Manivannan, P.; Kishorekumar, A.; Somasundaram, R.; Panneerselvam, R. Drought-induced biochemical modifications and proline metabolism in Abelmoschus esculentus (L.) Moench. Acta Bot. Croat. 2007, 66, 43–56. [Google Scholar]
- El-Tayeb, M.; El-Enany, A.; Ahmed, N. Salicylic acid-induced adaptive response to copper stress in sunflower (Helianthus annuus L.). Plant Growth Regul. 2006, 50, 191–199. [Google Scholar] [CrossRef]
- Hussein, M.; Balbaa, L.; Gaballah, M. Salicylic acid and salinity effects on growth of maize plants. Res. J. Agric. Biol. Sci. 2007, 3, 321–328. [Google Scholar]
- Abd Allah, M.M.S.; El-Bassiouny, H.M.S.; Elewa, T.A.E.; El-Sebai, T.N. Effect of salicylic acid and benzoic acid on growth, yield and some biochemical aspects of quinoa plant grown in sandy soil. Int. J. Chem. Tech. Res. 2015, 8, 216–225. [Google Scholar]
- Khodary, S. Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Int. J. Agric. Biol. 2004, 6, 5–8. [Google Scholar]
- Balibrea, M.E.; Dell’Amico, J.; Bolaŕn, M.C.; Pérez-Alfocea, F. Carbon partitioning and sucrose metabolism in tomato plants growing under salinity. Physiol. Plant. 2000, 110, 503–511. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Jensen, R.G. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 1996, 14, 89–97. [Google Scholar] [CrossRef]
- Bakry, B.A.; El-Hariri, D.M.; Sadak, M.S.; El-Bassiouny, H.M.S. Drought stress mitigation by foliar application of salicylic acid in two linseed varieties grown under newly reclaimed sandy soil. J. Appl. Sci. Res. 2012, 8, 3503–3514. [Google Scholar]
- Nazar, R.; Umar, S.; Khan, N.; Sareer, O. Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S. Afr. J. Bot. 2015, 98, 84–94. [Google Scholar] [CrossRef]
- Nazar, R.; Umar, S.; Khan, N.A. Exogenous salicylic acid improves photosynthesis and growth through increase in ascorbate-glutathione metabolism and S assimilation in mustard under salt stress. Plant Signal. Behav. 2015, 10, e1003751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, S.; Yang, S.; Chen, Y. Salicylic acid alleviates cadmium-induced inhibition of growth and photosynthesis through upregulating antioxidant defense system in two melon cultivars (Cucumis melo L.). Protoplasma 2015, 252, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Kotapati, K.V.; Palaka, B.K.; Ampasala, D.R. Alleviation of nickel toxicity in finger millet (Eleusine coracana L.) germinating seedlings by exogenous application of salicylic acid and nitric oxide. Crop J. 2017, 5, 240–250. [Google Scholar] [CrossRef]
- Chandrakar, V.; Dubey, A.; Keshavkant, S. Modulation of antioxidant enzymes by salicylic acid in arsenic exposed Glycine max L. J. Soil Sci. Plant Nutr. 2016, 16, 662–676. [Google Scholar] [CrossRef]
- Ghasemi, M.; Modarresi, M.; Babaeian Jelodar, N.; Bagheri, N.; Jamali, A. The evaluation of exogenous application of salicylic acid on physiological characteristics, proline and essential oil content of chamomile (Matricaria chamomilla L.) under normal and heat stress conditions. Agriculture 2016, 6, 31. [Google Scholar] [CrossRef]
- Zhou, Z.S.; Guo, K.; Elbaz, A.A.; Yang, Z.M. Salicylic acid alleviates mercury toxicity by preventing oxidative stress in roots of Medicago sativa. Environ. Exp. Bot. 2009, 65, 27–34. [Google Scholar] [CrossRef]
- Gaballah, M.; Rady, M. Salicylic acid mitigated cadmium toxicity by attenuating the oxidative stress in pea (Pisum sativum L.) plants. Int. J. Biol. Ecol. Environ. Sci. 2012, 1, 159–165. [Google Scholar]
- Manaa, A.; Gharbi, E.; Mimouni, H.; Wasti, S.; Aschi-Smiti, S.; Lutts, S.; Ahmed, H.B. Simultaneous application of salicylic acid and calcium improves salt tolerance in two contrasting tomato (Solanum lycopersicum) cultivars. S. Afr. J. Bot. 2014, 95, 32–39. [Google Scholar] [CrossRef]
- Munir, M.; Shabbir, G. Salicylic acid mediated heat stress tolerance in selected bread wheat genotypes of Pakistan. Pak. J. Bot. 2018, 50, 2141–2146. [Google Scholar]
- Kareem, F.; Rihan, H.; Fuller, M. The effect of exogenous applications of salicylic acid and molybdenum on the tolerance of drought in wheat. Agric. Res. Technol. 2017, 9, 555768. [Google Scholar] [CrossRef]
- Zengin, F. Effects of exogenous salicylic acid on growth characteristics and biochemical content of wheat seeds under arsenic stress. J. Environ. Biol. 2015, 36, 249. [Google Scholar] [PubMed]
- Al-Hakimi, A.B.M.; Hama, A.M. Ascorbic acid, thiamine or salicylic acid induced changes in some physiological parameters in wheat grown under copper stress. Plant Prot. Sci. 2011, 47, 92–108. [Google Scholar] [CrossRef]
- Li, Z.-G. Synergistic effect of antioxidant system and osmolyte in hydrogen sulfide and salicylic acid crosstalk-induced heat tolerance in maize (Zea mays L.) seedlings. Plant Signal. Behav. 2015, 10, e1051278. [Google Scholar] [CrossRef] [PubMed]
- Mohsenzadeh, S.; Shahrtash, M.; Mohabatkar, H. Interactive effects of salicylic acid and silicon on some physiological responses of cadmium-stressed maize seedlings. Iran. J. Sci. Technol. 2011, 35, 57–60. [Google Scholar]
- Krantev, A.; Yordanova, R.; Janda, T.; Szalai, G.; Popova, L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol. 2008, 165, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Pavlů, J.; Novák, J.; Koukalová, V.; Luklová, M.; Brzobohatý, B.; Černý, M. Cytokinin at the Crossroads of Abiotic Stress Signalling Pathways. Int. J. Mol. Sci. 2018, 19, 2450. [Google Scholar] [CrossRef] [PubMed]
- Kieber, J.J.; Schaller, G.E. The perception of cytokinin: A story 50 years in the making. Plant Physiol. 2010, 154, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.E.; Street, I.H.; Kieber, J.J. Cytokinin and the cell cycle. Curr. Opin. Plant Biol. 2014, 21, 7–15. [Google Scholar] [CrossRef]
- Su, Y.-H.; Liu, Y.-B.; Zhang, X.-S. Auxin–cytokinin interaction regulates meristem development. Mol. Plant 2011, 4, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Swarup, R.; Bennett, M.; Schaller, G.E.; Kieber, J.J. Cytokinin induces cell division in the quiescent center of the Arabidopsis root apical meristem. Curr. Biol. 2013, 23, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Bielach, A.; Podlešáková, K.; Marhavý, P.; Duclercq, J.; Cuesta, C.; Müller, B.; Grunewald, W.; Tarkowski, P.; Benková, E. Spatiotemporal regulation of lateral root organogenesis in Arabidopsis by cytokinin. Plant Cell 2012, 24, 3967–3981. [Google Scholar] [CrossRef] [PubMed]
- Bishopp, A.; Help, H.; El-Showk, S.; Weijers, D.; Scheres, B.; Friml, J.; Benková, E.; Mähönen, A.P.; Helariutta, Y. A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Curr. Biol. 2011, 21, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Efroni, I.; Han, S.-K.; Kim, H.J.; Wu, M.-F.; Steiner, E.; Birnbaum, K.D.; Hong, J.C.; Eshed, Y.; Wagner, D. Regulation of leaf maturation by chromatin-mediated modulation of cytokinin responses. Dev. Cell 2013, 24, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Durán-Medina, Y.; Díaz-Ramírez, D.; Marsch-Martínez, N. Cytokinins on the Move. Front. Plant Sci. 2017, 8, 146. [Google Scholar] [CrossRef] [Green Version]
- Zwack, P.J.; Rashotte, A.M. Cytokinin inhibition of leaf senescence. Plant Signal. Behav. 2013, 8, e24737. [Google Scholar] [CrossRef]
- Albacete, A.; Ghanem, M.E.; Martínez-Andújar, C.; Acosta, M.; Sánchez-Bravo, J.; Martínez, V.; Lutts, S.; Dodd, I.C.; Pérez-Alfocea, F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 2008, 59, 4119–4131. [Google Scholar] [CrossRef]
- Alvarez, S.; Marsh, E.L.; Schroeder, S.G.; Schachtman, D.P. Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant Cell Environ. 2008, 31, 325–340. [Google Scholar] [CrossRef]
- Shen, X.; Wang, Z.; Song, X.; Xu, J.; Jiang, C.; Zhao, Y.; Ma, C.; Zhang, H. Transcriptomic profiling revealed an important role of cell wall remodeling and ethylene signaling pathway during salt acclimation in Arabidopsis. Plant Mol. Biol. 2014, 86, 303–317. [Google Scholar] [CrossRef]
- Szabados, L.; Savoure, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Rajendrakumar, C.S.; Reddy, B.V.; Reddy, A.R. Proline-protein interactions: Protection of structural and functional integrity of M4 lactate dehydrogenase. Biochem. Biophy. Res. Commun. 1994, 201, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Dubey, R.S. Inhibition of ribonuclease and protease activities in arsenic exposed rice seedlings: Role of proline as enzyme protectant. J. Plant Physiol. 2006, 163, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.C.; McElwain, E.F.; Bohnert, H.J. Convergent induction of osmotic stress-responses: Abscisic acid, cytokinin, and the effects of NaCl. Plant Physiol. 1992, 100, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Türkan, I.; Demiral, T. Recent developments in understanding salinity tolerance. Environ. Exp. Bot. 2009, 67, 2–9. [Google Scholar] [CrossRef]
- Lokhande, V.H.; Suprasanna, P. Prospects of halophytes in understanding and managing abiotic stress tolerance. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: Berlin, Germany, 2012; pp. 29–56. [Google Scholar]
- Fitzgerald, T.L.; Waters, D.L.; Henry, R.J. Betaine aldehyde dehydrogenase in plants. Plant Biol. 2009, 11, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, G.V.; Wheeler, R.M.; Levine, L.H.; Stutte, G.W. Glycine betaine accumulation, ionic and water relations of red-beet at contrasting levels of sodium supply. J. Plant Physiol. 2001, 158, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Huner, N.P.; Öquist, G.; Sarhan, F. Energy balance and acclimation to light and cold. Trends Plant Sci. 1998, 3, 224–230. [Google Scholar] [CrossRef]
- Rathinasabapathi, B.; Fouad, W.M.; Sigua, C.A. beta-Alanine betaine synthesis in the Plumbaginaceae. Purification and characterization of a trifunctional, S-adenosyl-L-methionine-dependent N-methyltransferase from Limonium latifolium leaves. Plant Physiol. 2001, 126, 1241–1249. [Google Scholar] [CrossRef]
- Hansen, H.; Dörffling, K. Root-derived trans-zeatin riboside and abscisic acid in drought-stressed and rewatered sunflower plants: Interaction in the control of leaf diffusive resistance? Funct. Plant Biol. 2003, 30, 365–375. [Google Scholar] [CrossRef]
- Davies, W.J.; Kudoyarova, G.; Hartung, W. Long-distance ABA signaling and its relation to other signaling pathways in the detection of soil drying and the mediation of the plant’s response to drought. J. Plant Growth Regul. 2005, 24, 285. [Google Scholar] [CrossRef]
- Galston, A.W. Polyamines as modulators of plant development. Bioscience 1983, 33, 382–388. [Google Scholar] [CrossRef]
- Wimalasekera, R.; Scherer, G.F. Polyamines and cytokinin: Is nitric oxide biosynthesis the key to overlapping functions? In Nitric Oxide in Plant Physiology; Hayat, S., Mori, M., Pichtel, J., Ahmad, A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 65–76. [Google Scholar]
- Suresh, M.R.; Ramakrishna, S.; Adiga, P.R. Regulation of arginine decarboxylase and putrescine levels in Cucumis sativus cotyledons. Phytochemistry 1978, 17, 57–63. [Google Scholar] [CrossRef]
- Choudhuri, M.M.; Ghosh, B. Purification and partial characterization of arginine decarboxylase from rice embryos (Oryza sativa L.). Agric. Biol. Chem. 1982, 46, 739–743. [Google Scholar] [CrossRef]
- Palavan, N.; Goren, R.; Galston, A.W. Effects of some growth regulators on polyamine biosynthetic enzymes in etiolated pea seedlings. Plant Cell Physiol. 1984, 25, 541–546. [Google Scholar]
- Legocka, J.; Żarnowska, A. Role of polyamines in the cytokinin-dependent physiological processes II. Modulation of polyamine levels during cytokinin-stimulated expansion of cucumber cotyledons. Acta Physiol. Plant. 2000, 22, 395–401. [Google Scholar] [CrossRef]
- Sobieszczuk-Nowicka, E.; Rorat, T.; Legocka, J. Polyamine metabolism and S-adenosylmethionine decarboxylase gene expression during the cytokinin-stimulated greening process. Acta Physiol. Plant. 2007, 29, 495–502. [Google Scholar] [CrossRef]
- Yuanyuan, M.; Yali, Z.; Jiang, L.; Hongbo, S. Roles of plant soluble sugars and their responses to plant cold stress. Afr. J. Biotechnol. 2009, 8, 2004–2010. [Google Scholar]
- Hare, P.D.; Cress, W.A.; Van Staden, J. Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 1998, 21, 535–553. [Google Scholar] [CrossRef]
- Noiraud, N.; Maurousset, L.; Lemoine, R. Transport of polyols in higher plants. Plant Physiol. Biochem. 2001, 39, 717–728. [Google Scholar] [CrossRef]
- Lunn, J.E.; Delorge, I.; Figueroa, C.M.; Van Dijck, P.; Stitt, M. Trehalose metabolism in plants. Plant J. 2014, 79, 544–567. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H. Trehalose as a “chemical chaperone”: Fact and fantasy. Adv. Exp. Med. Biol. 2007, 594, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Loescher, W.H.; Tyson, R.H.; Everard, J.D.; Redgwell, R.J.; Bieleski, R.L. Mannitol Synthesis in Higher Plants: Evidence for the Role and Characterization of a NADPH-Dependent Mannose 6-Phosphate Reductase. Plant Physiol. 1992, 98, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Llanes, A.; Bertazza, G.; Palacio, G.; Luna, V. Different sodium salts cause different solute accumulation in the halophyte Prosopis strombulifera. Plant Biol. 2013, 15, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Bali, S.; Kaur, P.; Sharma, A.; Ohri, P.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Jasmonic acid-induced tolerance to root-knot nematodes in tomato plants through altered photosynthetic and antioxidative defense mechanisms. Protoplasma 2018, 255, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Per, T.S.; Khan, M.I.R.; Anjum, N.A.; Masood, A.; Hussain, S.J.; Khan, N.A. Jasmonates in plants under abiotic stresses: Crosstalk with other phytohormones matters. Environ. Exp. Bot. 2018, 145, 104–120. [Google Scholar] [CrossRef]
- Wasternack, C.; Parthier, B. Jasmonate-signalled plant gene expression. Trends Plant Sci. 1997, 2, 302–307. [Google Scholar] [CrossRef]
- Anjum, S.A.; Wang, L.; Farooq, M.; Khan, I.; Xue, L. Methyl Jasmonate-Induced Alteration in Lipid Peroxidation, Antioxidative Defence System and Yield in Soybean Under Drought. J. Agron. Crop Sci. 2011, 197, 296–301. [Google Scholar] [CrossRef]
- Shan, C.; Zhou, Y.; Liu, M. Nitric oxide participates in the regulation of the ascorbate-glutathione cycle by exogenous jasmonic acid in the leaves of wheat seedlings under drought stress. Protoplasma 2015, 252, 1397–1405. [Google Scholar] [CrossRef]
- Lehmann, J.; Atzorn, R.; Brückner, C.; Reinbothe, S.; Leopold, J.; Wasternack, C.; Parthier, B. Accumulation of jasmonate, abscisic acid, specific transcripts and proteins in osmotically stressed barley leaf segments. Planta 1995, 197, 156–162. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Farhangi-Abriz, S. Foliar sprays of salicylic acid and jasmonic acid stimulate H+-ATPase activity of tonoplast, nutrient uptake and salt tolerance of soybean. Ecotoxicol. Environ. Saf. 2018, 166, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Kumar, A.; Ashraf, M.; Akram, N.A. Salt-induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.). Afr. J. Biotechnol. 2012, 11, 2694–2703. [Google Scholar]
- Abdelgawad, Z.; Khalafaallah, A.A.; Abdallah, M. Impact of methyl jasmonate on antioxidant activity and some biochemical aspects of maize plant grown under water stress condition. Agric. Sci. 2014, 5, 1077. [Google Scholar] [CrossRef]
- Bandurska, H.; Stroiński, A.; Kubiś, J. The effect of jasmonic acid on the accumulation of ABA, proline and spermidine and its influence on membrane injury under water deficit in two barley genotypes. Acta Physiol. Plant. 2003, 25, 279–285. [Google Scholar] [CrossRef]
- Farooq, M.A.; Gill, R.A.; Islam, F.; Ali, B.; Liu, H.; Xu, J.; He, S.; Zhou, W. Methyl jasmonate regulates antioxidant defense and suppresses arsenic uptake in Brassica napus L. Front. Plant Sci. 2016, 7, 468. [Google Scholar] [CrossRef]
- Poonam, S.; Kaur, H.; Geetika, S. Effect of jasmonic acid on photosynthetic pigments and stress markers in Cajanus cajan (L.) Millsp. seedlings under copper stress. Am. J. Plant Sci. 2013, 4, 817. [Google Scholar] [CrossRef]
- Sirhindi, G.; Mir, M.A.; Abd-Allah, E.F.; Ahmad, P.; Gucel, S. Jasmonic acid modulates the physio-biochemical attributes, antioxidant enzyme activity, and gene expression in Glycine max under nickel toxicity. Front. Plant Sci. 2016, 7, 591. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Ahanger, M.A.; Alamri, S.A. Jasmonic acid alleviates negative impacts of cadmium stress by modifying osmolytes and antioxidants in faba bean (Vicia faba L.). Arch. Agron. Soil Sci. 2017, 63, 1889–1899. [Google Scholar] [CrossRef]
- Ali, E.; Hussain, N.; Shamsi, I.H.; Jabeen, Z.; Siddiqui, M.H.; Jiang, L.-X. Role of jasmonic acid in improving tolerance of rapeseed (Brassica napus L.) to Cd toxicity. J. Zhejiang Univ. Sci. B 2018, 19, 130–146. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Hamayun, M.; Lee, S.-K.; Lee, I.-J. Methyl jasmonate alleviated salinity stress in soybean. J. Crop Sci. Biotechnol. 2009, 12, 63–68. [Google Scholar] [CrossRef]
- Fedina, I.; Nedeva, D.; Georgieva, K.; Velitchkova, M. Methyl Jasmonate Counteract UV-B Stress in Barley Seedlings. J. Agron. Crop Sci. 2009, 195, 204–212. [Google Scholar] [CrossRef]
- Demkura, P.V.; Abdala, G.; Baldwin, I.T.; Ballaré, C.L. Jasmonate-Dependent and -Independent Pathways Mediate Specific Effects of Solar Ultraviolet B Radiation on Leaf Phenolics and Antiherbivore Defense. Plant Physiol. 2010, 152, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Front. Plant Sci. 2015, 6, 868. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Sarwat, M.; Bhat, N.A.; Wani, M.R.; Kazi, A.G.; Tran, L.S. Alleviation of cadmium toxicity in Brassica juncea L. (Czern. & Coss.) by calcium application involves various physiological and biochemical strategies. PLoS ONE 2015, 10, e0114571. [Google Scholar] [CrossRef]
- Carpena, R.O.; Vázquez, S.; Esteban, E.; Fernández-Pascual, M.; de Felipe, M.R.; Zornoza, P. Cadmium-stress in white lupin: Effects on nodule structure and functioning. Plant Physiol. Biochem. 2003, 41, 911–919. [Google Scholar] [CrossRef]
- Sakamoto, A.; Murata, N. The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant Cell Environ. 2002, 25, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Abass Ahanger, M.; Nasser Alyemeni, M.; Wijaya, L.; Alam, P.; Ashraf, M. Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J. Plant Interact. 2018, 13, 64–72. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Suzuki, T.; Fujita, M. Polyamine and nitric oxide crosstalk: Antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol. Environ. Saf. 2016, 126, 245–255. [Google Scholar] [CrossRef]
- Benavides, M.P.; Groppa, M.D.; Recalde, L.; Verstraeten, S.V. Effects of polyamines on cadmium- and copper-mediated alterations in wheat (Triticum aestivum L.) and sunflower (Helianthus annuus L.) seedling membrane fluidity. Arch. Biochem. Biophy. 2018, 654, 27–39. [Google Scholar] [CrossRef]
- Groppa, M.a.D.; Benavides, M.a.P.; Tomaro, M.a.L. Polyamine metabolism in sunflower and wheat leaf discs under cadmium or copper stress. Plant Sci. 2003, 164, 293–299. [Google Scholar] [CrossRef]
- Gális, I.; Šimek, P.; Narisawa, T.; Sasaki, M.; Horiguchi, T.; Fukuda, H.; Matsuoka, K. A novel R2R3 MYB transcription factor NtMYBJS1 is a methyl jasmonate-dependent regulator of phenylpropanoid-conjugate biosynthesis in tobacco. Plant J. 2006, 46, 573–592. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Xie, D.; Qi, D.; Peng, Q.; Chen, Z.; Schreiner, M.; Lin, Z.; Baldermann, S. Methyl Jasmonate-Induced Changes of Flavor Profiles During the Processing of Green, Oolong, and Black Tea. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.I.; Karimi, K.; Struik, P.C. Effect of exogenous application of methyl jasmonate on physiological and biochemical characteristics of Brassica napus L. cv. Talaye under salinity stress. S. Afr. J. Bot. 2018, 115, 5–11. [Google Scholar] [CrossRef]
- Fischer, C.; Höll, W. Food reserves of scots pine (Pinus sylvestris L.). Trees 1992, 6, 147–155. [Google Scholar] [CrossRef]
- El-Khallal, S. Some physiological roles of jasmonic acid in adaptation of pea seedlings to salt stress. Egypt. J. Biotechnol. 2001, 10, 249–271. [Google Scholar]
- Ghoulam, C.; Foursy, A.; Fares, K. Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ. Exp. Bot. 2002, 47, 39–50. [Google Scholar] [CrossRef]
- Harpreet, K.; Poonam, S.; Geetika, S. Sugar accumulation and its regulation by jasmonic acid in Brassica napus L. under salt stress. J. Stress Physiol. Biochem. 2013, 9, 53–64. [Google Scholar]
- Garcıa-Mata, C.; Lamattina, L. Nitric oxide and abscisic acid cross talk in guard cells. Plant Physiol. 2002, 128, 790–792. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Ann. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef]
- Hartung, W. The evolution of abscisic acid (ABA) and ABA function in lower plants, fungi and lichen. Funct. Plant Biol. 2010, 37, 806–812. [Google Scholar] [CrossRef]
- Xu, D.; Yuan, H.; Tong, Y.; Zhao, L.; Qiu, L.; Guo, W.; Shen, C.; Liu, H.; Yan, D.; Zheng, B. Comparative Proteomic Analysis of the Graft Unions in Hickory (Carya cathayensis) Provides Insights into Response Mechanisms to Grafting Process. Front. Plant Sci. 2017, 8, 676. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.M.S.; Ji, G.; Guo, H.; Zhao, L.; Zheng, B. Over-expression of a grafting-responsive gene from hickory increases abiotic stress tolerance in Arabidopsis. Plant Cell Rep. 2018, 37, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Ben Hassine, A.; Ghanem, M.E.; Bouzid, S.; Lutts, S. Abscisic acid has contrasting effects on salt excretion and polyamine concentrations of an inland and a coastal population of the Mediterranean xero-halophyte species Atriplex halimus. Ann. Bot. 2009, 104, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Pattanagul, W. Exogenous Abscisic Acid Enhances Sugar Accumulation in Rice. Asian J. Plant Sci. 2011, 10, 212–219. [Google Scholar]
- Sripinyowanich, S.; Klomsakul, P.; Boonburapong, B.; Bangyeekhun, T.; Asami, T.; Gu, H.; Buaboocha, T.; Chadchawan, S. Exogenous ABA induces salt tolerance in indica rice (Oryza sativa L.): The role of OsP5CS1 and OsP5CR gene expression during salt stress. Environ. Exp. Bot. 2013, 86, 94–105. [Google Scholar] [CrossRef]
- Karimi, R.; Ershadi, A. Role of exogenous abscisic acid in adapting of ‘Sultana’grapevine to low-temperature stress. Acta Physiol. Plant. 2015, 37, 151. [Google Scholar] [CrossRef]
- Ding, W.; Song, L.; Wang, X.; Bi, Y. Effect of abscisic acid on heat stress tolerance in the calli from two ecotypes of Phragmites communis. Biol. Plant. 2010, 54, 607–613. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, M.; Hu, J.; Zhang, X.; Wang, K.; Ashraf, M. Modulation Role of abscisic acid (ABA) on growth, water relations and glycinebetaine metabolism in two maize (Zea mays L.) cultivars under drought stress. Int. J. Mol. Sci. 2012, 13, 3189–3202. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Tajti, J.; Szalai, G.; Peeva, V.; Vegh, B.; Janda, T. Interaction of polyamines, abscisic acid and proline under osmotic stress in the leaves of wheat plants. Sci. Rep. 2018, 8, 12839. [Google Scholar] [CrossRef] [PubMed]
- Sarafraz-Ardakani, M.-R.; Khavari-Nejad, R.-A.; Moradi, F.; Najafi, F. Abscisic acid and cytokinin-induced osmotic and antioxidant regulation in two drought-tolerant and drought-sensitive cultivars of wheat during grain filling under water deficit in field conditions. Not. Sci. Biol. 2014, 6, 354–362. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushal, N.; Nayyar, H.; Gaur, P. Abscisic acid induces heat tolerance in chickpea (Cicer arietinum L.) seedlings by facilitated accumulation of osmoprotectants. Acta Physiol. Plant. 2012, 34, 1651–1658. [Google Scholar] [CrossRef]
- Lee, T.M.; Lur, H.S.; Chu, C. Role of abscisic acid in chilling tolerance of rice (Oryza sativa L.) seedlings.: II. Modulation of free polyamine levels. Plant Sci. 1997, 126, 1–10. [Google Scholar] [CrossRef]
- Marcinska, I.; Czyczylo-Mysza, I.; Skrzypek, E.; Grzesiak, M.T.; Janowiak, F.; Filek, M.; Dziurka, M.; Dziurka, K.; Waligorski, P.; Juzon, K.; et al. Alleviation of osmotic stress effects by exogenous application of salicylic or abscisic acid on wheat seedlings. Int. J. Mol. Sci. 2013, 14, 13171–13193. [Google Scholar] [CrossRef] [PubMed]
- Shevyakova, N.; Musatenko, L.; Stetsenko, L.; Vedenicheva, N.; Voitenko, L.; Sytnik, K.; Kuznetsov, V.V. Effects of abscisic acid on the contents of polyamines and proline in common bean plants under salt stress. Russ. J. Plant Physiol. 2013, 60, 200–211. [Google Scholar] [CrossRef]
- Planchet, E.; Verdu, I.; Delahaie, J.; Cukier, C.; Girard, C.; Morere-Le Paven, M.C.; Limami, A.M. Abscisic acid-induced nitric oxide and proline accumulation in independent pathways under water-deficit stress during seedling establishment in Medicago truncatula. J. Exp. Bot. 2014, 65, 2161–2170. [Google Scholar] [CrossRef] [PubMed]
- Verslues, P.E.; Bray, E.A. Role of abscisic acid (ABA) and Arabidopsis thaliana ABA-insensitive loci in low water potential-induced ABA and proline accumulation. J. Exp. Bot. 2006, 57, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Burbulis, N.; Jonytienė, V.; Kuprienė, R.; Blinstrubienė, A.; Liakas, V. Effect of abscisic acid on cold tolerance in Brassica napus shoots cultured in vitro. J. Food Agric. Environ. 2010, 8, 698–701. [Google Scholar]
- Yang, C.; Zhou, Y.; Fan, J.; Fu, Y.; Shen, L.; Yao, Y.; Li, R.; Fu, S.; Duan, R.; Hu, X.; et al. SpBADH of the halophyte Sesuvium portulacastrum strongly confers drought tolerance through ROS scavenging in transgenic Arabidopsis. Plant Physiol. Biochem. 2015, 96, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Golestan Hashemi, F.S.; Ismail, M.R.; Rafii, M.Y.; Aslani, F.; Miah, G.; Muharam, F.M. Critical multifunctional role of the betaine aldehyde dehydrogenase gene in plants. Biotechnol. Biotechnol. Equip. 2018, 32, 815–829. [Google Scholar] [CrossRef]
- Toumi, I.; Moschou, P.N.; Paschalidis, K.A.; Bouamama, B.; Salem-Fnayou, A.B.; Ghorbel, A.W.; Mliki, A.; Roubelakis-Angelakis, K.A. Abscisic acid signals reorientation of polyamine metabolism to orchestrate stress responses via the polyamine exodus pathway in grapevine. J. Plant Physiol. 2010, 167, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, R.A.; Hassanein, A.A.; El-din, A.B.; Salama, M.; Hashem, H.A. Role of jasmonic acid and abscisic acid treatments in alleviating the adverse effects of drought stress and regulating trypsin inhibitor production in soybean plant. Aust. J. Basic Appl. Sci. 2009, 3, 904–919. [Google Scholar]
- Jimenez-Bremont, J.F.; Ruiz, O.A.; Rodriguez-Kessler, M. Modulation of spermidine and spermine levels in maize seedlings subjected to long-term salt stress. Plant Physiol. Biochem. 2007, 45, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Rakitin, V.Y.; Prudnikova, O.; Rakitina, T.Y.; Karyagin, V.; Vlasov, P.; Novikova, G.; Moshkov, I. Interaction between ethylene and ABA in the regulation of polyamine level in Arabidopsis thaliana during UV-B stress. Russ. J. Plant Physiol. 2009, 56, 147–153. [Google Scholar] [CrossRef]
- Alcázar, R.; Cuevas, J.C.; Patron, M.; Altabella, T.; Tiburcio, A.F. Abscisic acid modulates polyamine metabolism under water stress in Arabidopsis thaliana. Physiol. Plant. 2006, 128, 448–455. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, M.Y.; Zhou, Y.F.; Liu, Y.L. Production of polyamines is enhanced by endogenous abscisic acid in maize seedlings subjected to salt stress. J. Integr. Plant Biol. 2005, 47, 1326–1334. [Google Scholar] [CrossRef]
- Hanzawa, Y.; Imai, A.; Michael, A.J.; Komeda, Y.; Takahashi, T. Characterization of the spermidine synthase-related gene family in Arabidopsis thaliana. FEBS Lett. 2002, 527, 176–180. [Google Scholar] [CrossRef]
- Urano, K.; Yoshiba, Y.; Nanjo, T.; Igarashi, Y.; Seki, M.; Sekiguchi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Characterization of Arabidopsis genes involved in biosynthesis of polyamines in abiotic stress responses and developmental stages. Plant Cell Environ. 2003, 26, 1917–1926. [Google Scholar] [CrossRef]
- Gurmani, A.; Bano, A.; Khan, S.; Din, J.; Zhang, J. Alleviation of salt stress by seed treatment with abscisic acid (ABA), 6-benzylaminopurine (BA) and chlormequat chloride (CCC) optimizes ion and organic matter accumulation and increases yield of rice (Oryza sativa L.). Aust. J. Crop Sci. 2011, 5, 1278. [Google Scholar]
- Gusta, L.; Trischuk, R.; Weiser, C. Plant cold acclimation: The role of abscisic acid. J. Plant Growth Regul. 2005, 24, 308–318. [Google Scholar] [CrossRef]
- Pelleschi, S.; Guy, S.; Kim, J.Y.; Pointe, C.; Mahe, A.; Barthes, L.; Leonardi, A.; Prioul, J.L. Ivr2, a candidate gene for a QTL of vacuolar invertase activity in maize leaves. Gene-specific expression under water stress. Plant Mol. Biol. 1999, 39, 373–380. [Google Scholar] [CrossRef]
- Trouverie, J.; Chateau-Joubert, S.; Thevenot, C.; Jacquemot, M.P.; Prioul, J.L. Regulation of vacuolar invertase by abscisic acid or glucose in leaves and roots from maize plantlets. Planta 2004, 219, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Trouverie, J.; Thévenot, C.; Rocher, J.P.; Sotta, B.; Prioul, J.L. The role of abscisic acid in the response of a specific vacuolar invertase to water stress in the adult maize leaf. J. Exp. Bot. 2003, 54, 2177–2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, U.; Bano, A. Effect of abscisic acid and chlorocholine chloride on nodulation and biochemical content of Vigna radiata L. under water stress. Pak. J. Bot. 2006, 38, 1511–1518. [Google Scholar]
- Kumar, S.; Kaur, G.; Nayyar, H. Exogenous application of abscisic acid improves cold tolerance in chickpea (Cicer arietinum L.). J. Agron. Crop Sci. 2008, 194, 449–456. [Google Scholar]
- Cheng, Z.; Jin, R.; Cao, M.; Liu, X.; Chan, Z. Exogenous application of ABA mimic 1 (AM1) improves cold stress tolerance in bermudagrass (Cynodon dactylon). Plant Cell Tissue Organ Cult. 2016, 125, 231–240. [Google Scholar] [CrossRef]
- An, Y.; Zhou, P.; Liang, J. Effects of exogenous application of abscisic acid on membrane stability, osmotic adjustment, photosynthesis and hormonal status of two lucerne (Medicago sativa L.) genotypes under high temperature stress and drought stress. Crop Pasture Sci. 2014, 65, 274–286. [Google Scholar] [CrossRef]
- Dias, M.C.; Oliveira, H.; Costa, A.; Santos, C. Improving elms performance under drought stress: The pretreatment with abscisic acid. Environ. Exp. Bot. 2014, 100, 64–73. [Google Scholar] [CrossRef]




| Plant Species | SA conc. | Abiotic Stress | Response of plants | Reference |
|---|---|---|---|---|
| Brassica juncea | 1 mM | Heavy metal (Pb- 0.25, 0.50, and 0.75 mM) | Content of proline, trehalose, and GB were increased in response to SA treatment | [96] |
| 1 mM | Heavy metal (Pb-0.25, 0.50, and 0.75 mM) | Levels of total carbohydrates and reducing sugar were enhanced significantly | [75] | |
| 0.5 mM | Water deficit | Activity of enzymes including γ-glutamyl kinase and proline oxidase were enhanced | [203] | |
| 0.5 mM | Salinity (100 mM) | Content of glutathione, an essential amino acid, was elevated | [204] | |
| 10−5 M | High Temperature | Levels of proline were significantly augmented | [182] | |
| Cucumis melo | 0.01, 0.05, 0.1 and 0.2 mM | Heavy Metal (Cd-50, 200, 400, and 800 µM) | Significant elevation in level of proline was observed by SA pre-treatment | [205] |
| Dianthus superbus | 0.5 mM | Salinity (0.3%, 0.6%, and 0.9%) | Proline content was enhanced by 53.7% under 0.3% salinity and 54.1% under 0.6% salinity | [183] |
| Eleucine coraccana | 0.2 mM | Heavy Metal (Ni-0.5 mM) | Level of proline was lowered in roots and shoots both | [206] |
| Glycine max | 1 mM | Salinity (4, 7, and 10 ds/m) | Lysine, methionine, isoleucine, and leucine contents were significantly enhanced. Phenylalanine and threonine levels were not influenced. Contents of non-essential amino acids viz. alanine, aspartic acid, glutamic acid, glycine, and serine were also augmented | [192] |
| 100, 200, 300 and 400 µM | Heavy Metal (As-10, 25, 50, 75, 100, and 125 µM) | Remarkable enhancement in proline levels was observed by SA dose | [207] | |
| Lens esculenta | 0.5 mM | Salinity (100 mM) | Activity of proline biosynthetic enzymes viz. γ-glutamyl kinase and pyrroline-5-carboxylate were significantly elevated. Similar enhancement in GB content was recorded. | [176] |
| Matricaria chamomilla | 1, 10, 25 and 100 mg | High Temperature | Free proline concentration was significantly increased | [208] |
| Medicago sativa | 0.2 mM | Heavy Metal (Mg-10 µM) | Proline levels were enhanced | [209] |
| Pisum sativum | 2 mM | Heavy Metal (Cd-0.75 and 1.5 mM) | Level of proline was further enhanced by SA supplementation | [210] |
| Solanum lycopersicum | 0.01 mM | Salinity (100 mM) | Soluble sugar and proline content were enhanced significantly | [211] |
| Torreya grandis | 0.5 mM | Salinity (0.2% and 0.4%) | Augmentation in proline content was observed in response to SA application | [181] |
| Triticum aestivum | 10−4 M | High Temperature | Proline level was elevated by 21% and sugar accumulation by 81% in field studies | [212] |
| 1.44 and 2.88 mM | Water deficit | Proline and GB content were increased in response to SA treatment | [213] | |
| 1 mM | Heavy Metal (As-50, 100, 150, 200, 250, 300, 350, and 400 µM) | Proline content was elevated by treatment of As stressed plants with SA | [214] | |
| 0.5 mM | High Temperature | Enhancement in proline content was observed | [23] | |
| 0.05 M | Water Deficit | Soluble sugars i.e., glucose, sucrose and total soluble sugars were elevated. Polysaccharides and total carbohydrate content were also enhanced. Elevation in glutamic acid, aspartic acid, leucine, tyrosine, alanine, and isoleucine etc., were elevated | [187] | |
| 100 ppm | Heavy Metal (Cu-5, 10, 20, and 40 mg L−1) | Soluble and total carbohydrates levels were enhanced by SA supplementation. Similar elevation in contents of proline and amino acid was observed | [215] | |
| Zea mays | 100 µM | High temperature | Proline, GB, and trehalose accumulation were increased | [216] |
| 500 µM | Heavy Metal (Cd-100 µM) | Total soluble sugar and proline levels were enhanced by SA application. | [217] | |
| 500 µM | Heavy Metal (Cd-10, 15, and 25 µM) | SA counter Cd stress by enhancing the levels of proline endogenously | [218] | |
| 10−2 M | Salinity (50, 100, and 150 mM) | Soluble sugar levels and polysaccharide content were recorded to be lowered | [199] |
| Plant Species | Stress | Impact on Osmolytes | Reference |
|---|---|---|---|
| Atriplex halimus | Salt | Increase in the contents of proline, glycine betaine, putrescine (Put), spermidine (Spd) and spermine (Spe). | [296] |
| Brassica napus | Cold | Increase in the contents of proline and soluble sugars. | [310] |
| Cicer arietinum | Heat | Increase in the contents of osmoprotectants like proline, glycine betaine and trehalose, accompanied by improved plant tolerance | [304] |
| Cold | Increase in the content of proline. | [327] | |
| Cynodon dactylon | Cold | Increase in the contents of proline accompanied by improvement in antioxidative response. | [328] |
| Glycine max | Drought | Increase in content of Put, but Spe and Spd contents were decreased. | [314] |
| Medicago sativa | Drought | Increase in proline content of shoots. | [329] |
| Oryza sativa | Salt | Increased accumulation of proline accompanied by regulation of expression levels of genes encoding proline biosynthetic enzymes (P5CS and P5CR). | [298] |
| Drought | Increased accumulation of total soluble sugars. | [297] | |
| Salt | Increase in the contents of proline and soluble sugars. | [321] | |
| Phaseolus vulgaris | Salt | Increase in the contents of Put, Spd and Spe. | [307] |
| Phragmites communis | Heat | Increase in the contents of proline and total thiols accompanied by improvement in antioxidative response. | [300] |
| Triticum aestivum | Drought | Increase in the contents of proline, glycine betaine and total soluble sugars accompanied by reduction in oxidative stress. | [303] |
| Drought | ABA signaling regulated the metabolism of osmolytes like proline and polyamines. | [302] | |
| Drought | Contents of proline and soluble carbohydrates were increased, but increase was not significant. | [306] | |
| Ulmus minor | Drought | Increase in proline content. | [330] |
| Vigna radiata | Drought | Increase in proline and sugar contents. | [326] |
| Vitis vinifera | Drought | Increase in the contents of Put, Spd and Spe | [313] |
| Cold | Increase in the contents of proline and soluble carbohydrates. | [299] | |
| Zea mays | Drought | Increase in glycine betaine content accompanied by enhanced activity of betaine aldehyde dehydrogenase (BADH). | [301] |
| Salt | Endogenous ABA stimulated biosynthesis of polyamines. | [318] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones Regulate Accumulation of Osmolytes Under Abiotic Stress. Biomolecules 2019, 9, 285. https://doi.org/10.3390/biom9070285
Sharma A, Shahzad B, Kumar V, Kohli SK, Sidhu GPS, Bali AS, Handa N, Kapoor D, Bhardwaj R, Zheng B. Phytohormones Regulate Accumulation of Osmolytes Under Abiotic Stress. Biomolecules. 2019; 9(7):285. https://doi.org/10.3390/biom9070285
Chicago/Turabian StyleSharma, Anket, Babar Shahzad, Vinod Kumar, Sukhmeen Kaur Kohli, Gagan Preet Singh Sidhu, Aditi Shreeya Bali, Neha Handa, Dhriti Kapoor, Renu Bhardwaj, and Bingsong Zheng. 2019. "Phytohormones Regulate Accumulation of Osmolytes Under Abiotic Stress" Biomolecules 9, no. 7: 285. https://doi.org/10.3390/biom9070285