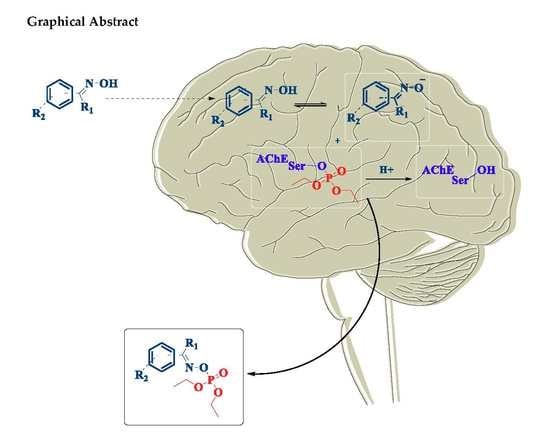
In Vitro Evaluation of Neutral Aryloximes as Reactivators for Electrophorus eel Acetylcholinesterase Inhibited by Paraoxon
Abstract
:1. Introduction
2. Experimental
2.1. General Information
2.2. Preparation of Test Solutions
2.3. Ellman’s Spectrophotometric Assays
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taylor, P. The cholinesterases. J. Biol. Chem. 1991, 266, 4025–4028. Available online: http://www.jbc.org/content/266/7/4025.long (accessed on 23 August 2019). [PubMed]
- Taylor, P.; Radic, Z. The Cholinesterases: From Genes to Proteins. Annu. Rev. Pharmacol. Toxicol. 1994, 34, 281–320. [Google Scholar] [CrossRef] [PubMed]
- Soreq, H.; Seidman, S. Acetylcholinesterase—New roles for an old actor. Nat. Rev. Neurosci. 2001, 2, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Quinn, D.M. Acetylcholinesterase: Enzyme structure, reaction dynamics, and virtual transition states. Chem. Rev. 1987, 87, 955–979. [Google Scholar] [CrossRef]
- Chatonnet, A.; Lockridge, O. Comparison of butyrylcholinesterase and acetylcholinesterase. Biochem. J. 1989, 260, 625–634. [Google Scholar] [CrossRef]
- Sussman, J.L.; Silman, I. Acetylcholinesterase: Structure and use as a model for specific cation—protein interactions. Curr. Opin. Struct. Boil. 1992, 2, 721–729. [Google Scholar] [CrossRef]
- Dvir, H.; Silman, I.; Harel, M.; Rosenberry, T.L.; Sussman, J.L. Acetylcholinesterase: From 3D Structure to Function. Chem. Interactions 2010, 187, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M.; Buckley, N.A.; Eyer, P.; Dawson, A.H. Management of acute organophosphorus pesticide poisoning. Lancet 2008, 371, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Costanzi, S.; Machado, J.-H.; Mitchell, M. Nerve Agents: What They Are, How They Work, How to Counter Them. ACS Chem. Neurosci. 2018, 9, 873–885. [Google Scholar] [CrossRef]
- Organisation for the Prohibition of Chemical Weapons—OPCW. Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on Their Destruction—CWC. 1997. Available online: www.opcw.org (accessed on 6 October 2019).
- Bernardes, M.F.F.; Pazin, M.; Pereira, L.C.; Dorta, D.J. Impact of Pesticides on Environmental and Human Health. Available online: https://www.intechopen.com/books/toxicology-studies-cells-drugs-and-environment/impact-of-pesticides-on-environmental-and-human-health (accessed on 23 August 2019).
- Pimentel, D. ‘Environmental and Economic Costs of the Application of Pesticides Primarily in the United States’. Environ. Dev. Sustain. 2005, 7, 229–252. [Google Scholar] [CrossRef]
- Ecobichon, D.J. Pesticide use in developing countries. Toxicology 2001, 160, 27–33. [Google Scholar] [CrossRef]
- Atreya, K.; Sitaula, B.K.; Johnsen, F.H.; Bajracharya, R.S. Continuing Issues in the Limitations of Pesticide Use in Developing Countries. J. Agric. Environ. Ethics 2011, 24, 49–62. [Google Scholar] [CrossRef]
- The Guardian. “Hundreds of new pesticides approved in Brazil under Bolsonaro”. Available online: https://www.theguardian.com/environment/2019/jun/12/hundreds-new-pesticides-approved-brazil-under-bolsonaro (accessed on 23 August 2019).
- Reuters. “Brazil approves rules for pesticides easing toxicity criteria”. Available online: https://www.reuters.com/article/us-brazil-pesticides/brazil-approves-rules-for-pesticides-easing-toxicity-criteria-idUSKCN1UI2JJ (accessed on 23 August 2019).
- Pignati, W.A.; Lima, F.A.N.S.; Lara, S.S.; Correa, M.L.M.; Barbosa, J.R.; Leão, L.H.C.; Pignatti, M.G. Spatial distribution of pesticide use in Brazil: A strategy for Health Surveillance. Ciência Saúde Coletiva 2017, 22, 3281–3293. [Google Scholar] [CrossRef]
- Dasgupta, S.; Mamingi, N.; Meisner, C. Pesticide use in Brazil in the era of agroindustrialization and globalization. Environ. Dev. Econ. 2001, 6, 459–482. [Google Scholar] [CrossRef] [Green Version]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Heal. 2016, 4, 231. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M.; Karalliedde, L.; Buckley, N.; Fernando, R.; Hutchinson, G.; Isbister, G.; Konradsen, F.; Murray, D.; Piola, J.C.; Senanayake, N.; et al. Pesticide poisoning in the developing world—A minimum pesticides list. Lancet 2002, 360, 1163–1167. [Google Scholar] [CrossRef]
- Piccoli, C.; Cremonese, C.; Koifman, R.; Koifman, S.; Freire, C. Occupational exposure to pesticides and hematological alterations: A survey of farm residents in the South of Brazil. Ciência Saúde Coletiva 2019, 24, 2325–2340. [Google Scholar] [CrossRef]
- Bardin, P.G.; van Eeden, S.F.; Moolman, J.A.; Foden, A.P.; Joubert, J.R. Organophosphate and Carbamate Poisoning. Arch. Intern. Med. 1994, 154, 1433–1441. [Google Scholar] [CrossRef]
- Steenland, K. Chronic neurological effects of organophosphate pesticides. BMJ 1996, 312, 1312–1313. [Google Scholar] [CrossRef] [Green Version]
- Eskenazi, B.; Bradman, A.; Castorina, R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ. Heal. Perspect. 1999, 107, 409–419. [Google Scholar] [CrossRef]
- Kofman, O.; Berger, A.; Massarwa, A.; Friedman, A.; Abu Jaffar, A. Motor Inhibition and Learning Impairments in School-Aged Children Following Exposure to Organophosphate Pesticides in Infancy. Pediatr. Res. 2006, 60, 88–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasiani, J.O.; Torres, P.; Silva, J.R.; Diniz, B.Z.; Caldas, E.D. Knowledge, Attitudes, Practices and Biomonitoring of Farmers and Residents Exposed to Pesticides in Brazil. Int. J. Environ. Res. Public Health 2012, 9, 3051–3068. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, S.K.; Tripathi, S.; Ravishanker, D. A study of neurologic symptoms on exposure to organophosphate pesticides in the children of agricultural workers. Indian J. Occup. Environ. Med. 2010, 14, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Mercey, G.; Verdelet, T.; Renou, J.; Kliachyna, M.; Baati, R.; Nachon, F.; Jean, L.; Renard, P.-Y. Reactivators of Acetylcholinesterase Inhibited by Organophosphorus Nerve Agents. Accounts Chem. Res. 2012, 45, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, M.H. Estimates of acute pesticide poisoning in agricultural workers in less developed countries. Toxicol. Rev. 2005, 24, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Jeffries, C.L.; Walker, T. Biological Control of Mosquito Vectors: Past, Present, and Future. Insects 2016, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.A.; Urbinatti, P.R.; Sallum, M.A.M.; Kuniy, A.A.; Moresco, G.G.; Fernandes, A.; Nagaki, S.S.; Natal, D. Brazilian mosquito (Diptera: Culicidae) fauna: I. Anopheles species from Porto Velho, Rondônia state, western Amazon, Brazil. Revista do Instituto de Medicina Tropical de São Paulo 2012, 54, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, B.D.; Tadei, W.P. Malaria vectors in the Brazilian Amazon: Anopheles of the subgenus Nyssorhynchus. Revista do Instituto de Medicina Tropical de São Paulo 2000, 42, 87–94. [Google Scholar]
- Corbel, V.; Achee, N.L.; Chandre, F.; Coulibaly, M.B.; Dusfour, I.; Fonseca, D.M.; Grieco, J.; Juntarajumnong, W.; Lenhart, A.; Martins, A.J.; et al. Tracking Insecticide Resistance in Mosquito Vectors of Arboviruses: The Worldwide Insecticide resistance Network (WIN). PLOS Neglected Trop. Dis. 2016, 10, e0005054. [Google Scholar] [CrossRef]
- Achee, N.L.; Grieco, J.P.; Vatandoost, H.; Seixas, G.; Pinto, J.; Ching-Ng, L.; Martins, A.J.; Juntarajumnong, W.; Corbel, V.; Gouagna, C.; et al. Alternative strategies for mosquito-borne arbovirus control. PLOS Neglected Trop. Dis. 2019, 13, e0006822. [Google Scholar]
- Peters, R.A. Croonian Lecture—Lethal synthesis. Proc. R. Soc. London. Ser. B: Boil. Sci. 1952, 139, 143–170. [Google Scholar]
- Yanagisawa, N. [The nerve agent sarin: History, clinical manifestations, and treatment]. Brain Nerve 2014, 66, 561–569. [Google Scholar] [PubMed]
- Antonijević, B.; Stojiljkovic, M.P. Unequal Efficacy of Pyridinium Oximes in Acute Organophosphate Poisoning. Clin. Med. Res. 2007, 5, 71–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aroniadou-Anderjaska, V.; Figueiredo, T.H.; Apland, J.P.; Prager, E.M.; Pidoplichko, V.I.; Miller, S.L.; Braga, M.F.M. Long-term neuropathological and behavioral impairments after exposure to nerve agents. Ann. N. Y. Acad. Sci. 2006, 1374, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Aroniadou-Anderjaska, V.; Figueiredo, T.H.; Apland, J.P.; Qashu, F.; Braga, M.F. Primary brain targets of nerve agents: The role of the amygdala in comparison to the hippocampus. NeuroToxicology 2009, 30, 772–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moshiri, M.; Darchini-Maragheh, E.; Balali-Mood, M. Advances in toxicology and medical treatment of chemical warfare nerve agents. DARU J. Pharm. Sci. 2012, 20, 81. [Google Scholar] [CrossRef] [PubMed]
- Čolović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Wilson, I.B.; Ginsburg, S. Reactivation of alkylphosphate inhibited acetylcholinesterase by bis quaternary derivatives of 2-PAM and 4-PAM. Biochem. Pharmacol. 1959, 1, 200–206. [Google Scholar] [CrossRef]
- Cannard, K.J. The acute treatment of nerve agent exposure. Neurol. Sci. 2006, 249, 86–94. [Google Scholar] [CrossRef]
- Kuca, K.; Jun, D.; Musilek, K. Structural Requirements of Acetylcholinesterase Reactivators. Mini-Reviews Med. Chem. 2006, 6, 269–277. [Google Scholar] [CrossRef]
- Worek, F.; Wille, T.; Koller, M.; Thiermann, H. Structural requirements for effective oximes – Evaluation of kinetic in vitro data with phosphylated human AChE and structurally different oximes. Chem. Interactions 2013, 203, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Milatovic, D.; Jokanović, M. Pyridinium Oximes as Cholinesterase Reactivators in the Treatment of OP Poisoning. In Handbook of Toxicology of Chemical Warfare Agents; Elsevier BV: San Diego, CA, USA, 2009; pp. 985–996. [Google Scholar]
- Saint-André, G.; Kliachyna, M.; Kodepelly, S.; Louise-Leriche, L.; Gillon, E.; Renard, P.-Y.; Nachon, F.; Baati, R.; Wagner, A. Design, synthesis and evaluation of new α-nucleophiles for the hydrolysis of organophosphorus nerve agents: Application to the reactivation of phosphorylated acetylcholinesterase. Tetrahedron 2011, 67, 6352–6361. [Google Scholar] [CrossRef]
- Sit, R.K.; Radić, Z.; Gerardi, V.; Zhang, L.; Garcia, E.; Katalinić, M.; Amitai, G.; Kovarik, Z.; Fokin, V.V.; Sharpless, K.B.; et al. New Structural Scaffolds for Centrally Acting Oxime Reactivators of Phosphylated Cholinesterases*. J. Boil. Chem. 2011, 286, 19422–19430. [Google Scholar] [CrossRef] [PubMed]
- Musilek, K.; Kuca, K.; Jun, D.; Dohnal, V.; Dolezal, M. Synthesis of the novel series of bispyridinium compounds bearing (E)-but-2-ene linker and evaluation of their reactivation activity against chlorpyrifos-inhibited acetylcholinesterase. Bioorganic Med. Chem. Lett. 2006, 16, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Timperley, C.M.; Banks, R.E.; Young, I.M.; Haszeldine, R.N. Synthesis of some fluorine-containing pyridinealdoximes of potential use for the treatment of organophosphorus nerve-agent poisoning. J. Fluor. Chem. 2011, 132, 541–547. [Google Scholar] [CrossRef]
- Jokanovic, M. Structure-Activity Relationship and Efficacy of Pyridinium Oximes in the Treatment of Poisoning with Organophosphorus Compounds: A Review of Recent Data. Curr. Top. Med. Chem. 2012, 12, 1775–1789. [Google Scholar] [CrossRef]
- Acharya, J.; Gupta, A.K.; Mazumder, A.; Dubey, D.K. In-vitro regeneration of sarin inhibited electric eel acetylcholinesterase by bis-pyridinium oximes bearing xylene linker. Eur. J. Med. Chem. 2009, 44, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.; Dubey, D.K.; Srivastava, A.K.; Raza, S.K. In vitro reactivation of sarin-inhibited human acetylcholinesterase (AChE) by bis-pyridinium oximes connected by xylene linkers. Toxicol. Vitr. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Musilek, K.; Jun, D.; Cabal, J.; Kassa, J.; Gunn-Moore, F.; Kuca, K. Design of a Potent Reactivator of Tabun-Inhibited AcetylcholinesteraseSynthesis and Evaluation of (E)-1-(4-Carbamoylpyridinium)-4-(4-hydroxyiminomethylpyridinium)-but-2-ene Dibromide (K203). J. Med. Chem. 2007, 50, 5514–5518. [Google Scholar] [CrossRef]
- Cavalcante, S.F.A.; Kitagawa, D.A.S.; Rodrigues, R.B.; Bernardo, L.B.; da Silva, T.N.; dos Santos, W.V.; Correa, A.B.A.; de Almeida, J.S.F.D.; França, T.C.C.; Kuča, K.; et al. Synthesis and in vitro evaluation of neutral aryloximes as reactivators of Electrophorus eel Acetylcholinesterase inhibited by NEMP, a VX surrogate. Chem. Biol. Interact. 2019, 309. in press. [Google Scholar] [CrossRef]
- Sahu, A.K.; Sharma, R.; Gupta, B.; Musilek, K.; Kuca, K.; Acharya, J.; Ghosh, K.K. Oxime-mediated in vitro reactivation kinetic analysis of organophosphates-inhibited human and electric eel acetylcholinesterase. Toxicol. Mech. Methods 2016, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Worek, F.; Reiter, G.; Eyer, P.; Szinicz, L. Reactivation kinetics of acetylcholinesterase from different species inhibited by highly toxic organophosphates. Arch. Toxicol. 2002, 76, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.S.; Prates, A.; Alves, S.R.; Oliveira-Silva, J.J.; Riehl, C.A.S.; Figueroa-Villar, J.D. The effect of neutral oximes on the reactivation of human acetylcholinesterase inhibited with paraoxon. J. Braz. Chem. Soc. 2012, 23, 1216–1225. [Google Scholar] [CrossRef] [Green Version]
- Ellman, G.L.; Courtney, K.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Cavalcante, S.; Kitagawa, D.; Rodrigues, R.; Cardozo, M.; Paula, R.; Correa, A.B.; Simas, A. Straightforward, economical procedures for microscale ellman’s test for cholinesterase inhibition and reactivation. Química Nova 2018, 41, 1192–1195. [Google Scholar] [CrossRef]
- Pohanka, M.; Hrabinová, M.; Kuca, K.; Simonato, J.-P. Assessment of Acetylcholinesterase Activity Using Indoxylacetate and Comparison with the Standard Ellman’s Method. Int. J. Mol. Sci. 2011, 12, 2631–2640. [Google Scholar] [CrossRef]
- Sit, R.K.; Fokin, V.V.; Amitai, G.; Sharpless, K.B.; Taylor, P.; Radić, Z. Imidazole aldoximes effective in assisting butyrylcholinesterase catalysis of organophosphate detoxification. J. Med. Chem. 2014, 57, 1378–1389. [Google Scholar] [CrossRef]
- Radić, Z.; Sit, R.K.; Kovarik, Z.; Berend, S.; Garcia, E.; Zhang, L.; Amitai, G.; Green, C.; Radić, B.; Fokin, V.V.; et al. Refinement of Structural Leads for Centrally Acting Oxime Reactivators of Phosphylated Cholinesterases*. J. Boil. Chem. 2012, 287, 11798–11809. [Google Scholar] [CrossRef]
- Bajgar, J. Organophosphates/Nerve Agent Poisoning: Mechanism of Action, Diagnosis, Prophylaxis, And Treatment. Adv. Clin. Chem. 2004, 38, 151–216. [Google Scholar]
- Tattersall, J.E.H. Ion channel blockade by oximes and recovery of diaphragm muscle from soman poisoning in vitro. Br. J. Pharmacol. 1993, 108, 1006–1015. [Google Scholar] [CrossRef] [Green Version]
- Ozgun, D.O.; Yamali, C.; Gul, H.I.; Taslimi, P.; Gulcin, I.; Yanik, P.D.; Supuran, C.T. Inhibitory effects of isatin Mannich bases on carbonic anhydrases, acetylcholinesterase, and butyrylcholinesterase. J. Enzym. Inhib. Med. Chem. 2016, 31, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.P.; Rao, J.V. Biological evaluation of schiff bases of new isatin derivatives for anti alzheimer’s activity. Asian J. Pharm. Clin. Res. 2014, 7, 114–117. Available online: https://innovareacademics.in/journals/index.php/ajpcr/article/view/966 (accessed on 23 August 2019).
- Barcelos, R.P.; Lima Portella, R.; Lugokenski, T.H.; Rosa, E.J.F.; Amaral, G.P.; Garcia, L.F.M.; Bresolin, L.; Carratu, V.; Soares, F.A.A.; Vargas Barbosa, N.B. Isatin-3-N4-benzilthiosemicarbazone, a non-toxic thiosemicarbazone derivative, protects and reactivates rat and human cholinesterases inhibited by methamidophos in vitro and in silico. Toxicol. In Vitro 2012, 26, 1030–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boar, B.R.; Cross, A.J. Isatin Derivatives, Processes for the Preparation Thereof and Pharmacautical Composition Comprising the Same. WO 9312085, 24 June 1993. [Google Scholar]
- Bridges, T.M.; Marlo, J.E.; Niswender, C.M.; Jones, C.K.; Jadhav, S.B.; Gentry, P.R.; Plumley, H.C.; Weaver, C.D.; Conn, P.J.; Lindsley, C.W. Discovery of the first highly M5-preferring muscarinic acetylcholine receptor ligand, an M5 positive allosteric modulator derived from a series of 5-trifluoromethoxy N-benzyl isatins. J. Med. Chem. 2009, 52, 3445–3448. [Google Scholar] [CrossRef] [PubMed]
- De Paula, R.L.; De Almeida, J.S.F.D.; Cavalcante, S.F.A.; Gonçalves, A.S.; Simas, A.B.C.; Franca, T.C.C.; Valis, M.; Kuca, K.; Nepovimova, E.; Granjeiro, J.M. Molecular Modeling and In Vitro Studies of a Neutral Oxime as a Potential Reactivator for Acetylcholinesterase Inhibited by Paraoxon. Molecules 2018, 23, 2954. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, S.; Simas, A.; Kitagawa, D.; Bernardo, L.; Rodrigues, R.; Correa, A.; Paula, R.; Freitas, L.; Diz de Almeida, J.; França, T.; et al. Derivados da Indolin-2-ona e Seus Intermediários, Produtos, Métodos de Obtenção e Usos. BR1020180750046, 3 December 2018. [Google Scholar]
- Zorbaz, T.; Malinak, D.; Mariković, N.; Maček Hrvak, N.; Zandona, A.; Novotny, M.; Sharka, A.; Andrys, R.; Benkova, M.; Soukup, O.; et al. Pyridinium Oximes with Ortho-Positioned Chlorine Moiety Exhibit Improved Physicochemical Properties and Efficient Reactivation of Human Acetylcholinesterase Inhibited by Several Nerve Agents. J. Med. Chem. 2018, 61, 10753–10766. [Google Scholar] [CrossRef] [PubMed]
- Zorbaz, T.; Malinak, D.; Kuča, K.; Musilek, K.; Kovarik, Z. Butyrylcholinesterase inhibited by nerve agents is efficiently reactivated with chlorinated pyridinium oximes. Chem. Biol. Interact. 2019, 307, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Bassetto, M.; Ferla, S.; Pertusati, F. Polyfluorinated groups in medicinal chemistry. Futur. Med. Chem. 2015, 7, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Biffinger, J.C.; Kim, H.W.; DiMagno, S.G. The Polar Hydrophobicity of Fluorinated Compounds. ChemBioChem 2004, 5, 622–627. [Google Scholar] [CrossRef]







| Entry | Code | Name | pKa 1 | logP | Reactivator Concentration (µmol/L) | ||
|---|---|---|---|---|---|---|---|
| 1000 | 100 | 10 | |||||
| 1 | 22a | 2-hydroxybenzaldoxime | 6.61 (OH = 9.99) | 1.39 | 9 ± 1 | 4 ± 1 | 1 ± 0 |
| 2 | 22b | 3-hydroxybenzaldoxime | 7.09 (OH = 9.74) | 1.39 | 3 ± 0 | 3 ± 0 | 2 ± 0 |
| 3 | 22c | 4-hydroxybenzaldoxime | 7.57 (OH = 10.15) | 1.39 | 6 ± 1 | 6 ± 1 | 6 ± 1 |
| 4 | 22d | 2-methoxybenzaldoxime | 6.69 | 1.54 | 2 ± 0 | 2 ± 0 | 1 ± 0 |
| 5 | 22e | 3-methoxybenzaldoxime | 7.20 | 1.54 | 4 ± 0 | 1 ± 0 | 1 ± 0 |
| 6 | 22f | 4-methoxybenzaldoxime | 7.69 | 1.54 | 4 ± 1 | 2 ± 0 | 2 ± 0 |
| 7 | 22g | 2-bromobenzaldoxime | 6.96 | 2.46 | 3 ± 0 | 2 ± 0 | 2 ± 0 |
| 8 | 22h | 3-bromobenzaldoxime | 7.31 | 2.46 | 4 ± 0 | 1 ± 0 | 0 |
| 9 | 22i | 4-bromobenzaldoxime | 6.69 | 2.46 | 2 ± 0 | 2 ± 0 | 2 ± 0 |
| 10 | 22j | 2-chlorobenzaldoxime | 6.80 | 2.30 | 30 ± 2 | 7 ± 1 | 2 ± 0 |
| 11 | 22k | 3-chlorobenzaldoxime | 7.26 | 2.30 | 3 ± 0 | 2 ± 0 | 1 ± 0 |
| 12 | 22l | 4-chlorobenzaldoxime | 6.67 | 2.30 | 3 ± 0 | 3 ± 0 | 1 ± 0 |
| 13 | 22m | 2-fluorobenzaldoxime | 6.82 | 1.84 | 4 ± 0 | 1 ± 0 | 3 ± 0 |
| 14 | 22n | 3-fluorobenzaldoxime | 7.11 | 1.84 | 4 ± 0 | 2 ± 0 | 2 ± 1 |
| 15 | 22o | 4-fluorobenzaldoxime | 6.80 | 1.84 | 3 ± 0 | 2 ± 0 | 2 ± 0 |
| 16 | 22p | 2-trifluoromethyl benzaldoxime | 5.52 | 2.57 | 26 ± 3 | 5 ± 0 | 1 ± 0 |
| 17 | 22q | 3-trifluoromethyl benzaldoxime | 6.13 | 2.57 | 77 ± 4 | 5 ± 0 | 1 ± 0 |
| 18 | 22r | 4-trifluoromethyl benzaldoxime | 6.29 | 2.57 | 10 ± 1 | 4 ± 0 | 1 ± 0 |
| 19 | 22s | 2-methylbenzaldoxime | 8.08 | 2.21 | 6 ± 0 | 1 ± 0 | 1 ± 0 |
| 20 | 22t | 3-methylbenzaldoxime | 7.97 | 2.21 | 12 ± 2 | 1 ± 0 | 0 |
| 21 | 22u | 4-methylbenzaldoxime | 8.14 | 2.21 | 14 ± 2 | 0 | 0 |
| 22 | 22v | 4-isopropylbenzaldoxime | 8.21 | 2.94 | 2 ± 0 | 1 ± 0 | 2 ± 0 |
| 23 | 22w | 3-nitrobenzaldoxime | 5.83 | 1.64 | 6 ± 0 | 3 ± 0 | 5 ± 1 |
| 24 | 22x | 4-nitrobenzaldoxime | 5.80 | 1.64 | 8 ± 1 | 7 ± 1 | 4 ± 1 |
| 25 | 22y | 4-(N,N-dimethylamino) benzaldoxime | 8.71 | 1.80 | 7 ± 1 | 6 ± 0 | 2 ± 0 |
| 26 | 22z | 4-(N,N-diethylamino) benzaldoxime | 8.80 | 2.52 | 7 ± 1 | 5 ± 1 | 5 ± 1 |
| 27 | 22aa | Vanillin oxime | 7.18 (OH = 10.60) | 1.23 | 5 ± 0 | 4 ± 0 | 4 ± 1 |
| 28 | 22ab | Isovanillin oxime | 6.21 (OH = 10.47) | 1.23 | 10 ± 0 | 4 ± 0 | 4 ± 0 |
| 29 | 22ac | Orthovanillin oxime | 7.18 (OH = 10.14) | 1.23 | 22 ± 2 | 4 ± 0 | 4 ± 0 |
| 30 | 22ad | Pyridine-4-aldoxime | 10.21 | 0.48 | 6 ± 1 | 5 ± 0 | 1 ± 0 |
| 31 | 22ae | Pyridine-2-aldoxime | 9.02 | 1.15 | 9 ± 1 | 3 ± 0 | 0 |
| 32 | 22af | Isatin 3-oxime | 7.13 (NH = 15.51) | 0.96 | 67 ±10 | 15 ± 2 | 8 ± 0 |
| 33 | 22ag | N-benzylisatin 3-oxime | 7.31 | 2.55 | 85 ±10 | 9 ± 1 | 1 ± 0 |
| 34 | 14 | Pralidoxime (2-PAM) 2 | 7.63 | -3.26 | 39 ± 2 | 42 ± 2 | 16 ± 3 |
| 35 | 15 | Obidoxime (OBD) | 7.51, 8.11 | -6.93 | 62 ± 3 | 88 ± 3 | 58 ± 3 |
| 36 | 16 | Trimedoxime (TMB) | 8.63, 9.24 | -7.04 | 84 ± 5 | 75 ± 3 | 29 ± 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitagawa, D.A.S.; Cavalcante, S.F.d.A.; de Paula, R.L.; Rodrigues, R.B.; Bernardo, L.B.; da Silva, M.C.J.; da Silva, T.N.; dos Santos, W.V.; Granjeiro, J.M.; de Almeida, J.S.F.D.; et al. In Vitro Evaluation of Neutral Aryloximes as Reactivators for Electrophorus eel Acetylcholinesterase Inhibited by Paraoxon. Biomolecules 2019, 9, 583. https://doi.org/10.3390/biom9100583
Kitagawa DAS, Cavalcante SFdA, de Paula RL, Rodrigues RB, Bernardo LB, da Silva MCJ, da Silva TN, dos Santos WV, Granjeiro JM, de Almeida JSFD, et al. In Vitro Evaluation of Neutral Aryloximes as Reactivators for Electrophorus eel Acetylcholinesterase Inhibited by Paraoxon. Biomolecules. 2019; 9(10):583. https://doi.org/10.3390/biom9100583
Chicago/Turabian StyleKitagawa, Daniel A. S., Samir F. de A. Cavalcante, Reuel L. de Paula, Rafael B. Rodrigues, Leandro B. Bernardo, Munique C. J. da Silva, Thiago N. da Silva, Wellington V. dos Santos, José M. Granjeiro, Joyce S. F. D. de Almeida, and et al. 2019. "In Vitro Evaluation of Neutral Aryloximes as Reactivators for Electrophorus eel Acetylcholinesterase Inhibited by Paraoxon" Biomolecules 9, no. 10: 583. https://doi.org/10.3390/biom9100583