Are Inflamed Periodontal Tissues Endogenous Source of Advanced Glycation End-Products (AGEs) in Individuals with and without Diabetes Mellitus? A Systematic Review
Abstract
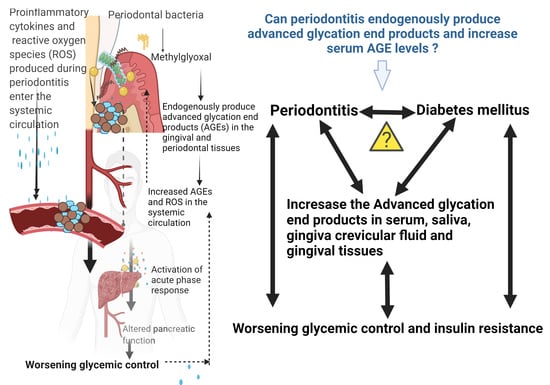
:1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Focus Question
2.3. Search Strategy, Information Sources, and Keywords
2.4. Inclusion and Exclusion Criteria
2.5. Study Screening and Data Extraction
- Methods: trial design, duration of the study, country of the study, type of study design, and ethics committee approval (if mentioned).
- Participants: number in each group, number analyzed, mean age, range, the gender of the participants; the severity of the condition, diagnostic criteria for diabetes and periodontitis (if mentioned).
- Exposure: duration of periodontitis and diabetes (if mentioned).
- The mean and standard deviation of the following outcomes:
- Concentration of AGEs or receptors for AGE (RAGE) in any form in saliva/GCF/serum/blood/gingiva tissues.
- Periodontal parameters: PD, CAL, BOP, GI, PI, the radiographic measure of bone loss (if present) as of all the outcomes.
- Fasting, random, and postprandial blood glucose levels; glycated hemoglobin (whichever is applicable); body mass index (BMI); microbial profile; method of analysis of AGE.
- Notes: Funding and potential conflicts of interest of authors in the study.
2.6. Risk of Bias (ROB) of Individual Studies
3. Results
3.1. Characteristics of the Included Studies
3.2. Detection of AGEs and RAGE in Periodontal Tissues and Oral Fluids
3.3. Detection of AGE, Receptors of RAGE and sRAGE in Serum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ebersole, J.L.; Holt, S.C.; Hansard, R.; Novak, M.J. Microbiologic and immunologic characteristics of periodontal disease in Hispanic americans with type 2 diabetes. J. Periodontol. 2008, 79, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Battino, M.; Bullon, P.; Wilson, M.; Newman, H. Oxidative injury and inflammatory periodontal diseases: The challenge of anti-oxidants to free radicals and reactive oxygen species. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 1999, 10, 458–476. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.A.; Macpherson, L.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Chapple, I.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S74–S84. [Google Scholar] [CrossRef]
- Cugini, C.; Ramasubbu, N.; Tsiagbe, V.K.; Fine, D.H. Dysbiosis from a Microbial and Host Perspective Relative to Oral Health and Disease. Front. Microbiol. 2021, 12, 617485. [Google Scholar] [CrossRef]
- Christina, P.; Velitchka, D.P.; Vladimir, P. Microbiology of Periodontal Diseases. A Review. Biotechnol. Biotechnol. Equip. 2013, 27, 3754–3759. [Google Scholar] [CrossRef]
- Chapple, I.L.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontology 2000 2007, 43, 160–232. [Google Scholar] [CrossRef]
- Meyle, J.; Chapple, I. Molecular aspects of the pathogenesis of periodontitis. Periodontology 2000 2015, 69, 7–17. [Google Scholar] [CrossRef]
- Folli, F.; Corradi, D.; Fanti, P.; Davalli, A.; Paez, A.; Giaccari, A.; Perego, C.; Muscogiuri, G. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: Avenues for a mechanistic-based therapeutic approach. Curr. Diabetes Rev. 2011, 7, 313–324. [Google Scholar] [CrossRef]
- Wang, Y.; Andrukhov, O.; Rausch-Fan, X. Oxidative Stress and Antioxidant System in Periodontitis. Front. Physiol. 2017, 8, 910. [Google Scholar] [CrossRef] [Green Version]
- Albandar, J.M. Global risk factors and risk indicators for periodontal diseases. Periodontology 2000 2002, 29, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Albandar, J.M.; Streckfus, C.F.; Adesanya, M.R.; Winn, D.M. Cigar, pipe, and cigarette smoking as risk factors for periodontal disease and tooth loss. J. Periodontol. 2000, 71, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. Periodontology 2000 2013, 62, 59–94. [Google Scholar] [CrossRef] [PubMed]
- Lalla, E.; Papapanou, P.N. Diabetes mellitus and periodontitis: A tale of two common interrelated diseases. Nat. Rev. Endocrinol. 2011, 7, 738–748. [Google Scholar] [CrossRef]
- Polak, D.; Sanui, T.; Nishimura, F.; Shapira, L. Diabetes as a risk factor for periodontal disease-plausible mechanisms. Periodontology 2000 2020, 83, 46–58. [Google Scholar] [CrossRef]
- Moeintaghavi, A.; Arab, H.R.; Bozorgnia, Y.; Kianoush, K.; Alizadeh, M. Non-surgical periodontal therapy affects metabolic control in diabetics: A randomized controlled clinical trial. Aust. Dent. J. 2012, 57, 31–37. [Google Scholar] [CrossRef]
- Chapple, I.L.; Genco, R. Working group 2 of joint EFP/AAP workshop Diabetes and periodontal diseases: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Clin. Periodontol. 2013, 40 (Suppl. 14), S106–S112. [Google Scholar] [CrossRef]
- Menezes, T.O.; Rodrigues, M.C.; Nogueira, B.M.; Menezes, S.A.; Silva, S.H.; Vallinoto, A.C. Oral and systemic manifestations in HIV-1 patients. Rev. Soc. Bras. Med. Trop. 2015, 48, 83–86. [Google Scholar] [CrossRef] [Green Version]
- Ryder, M.I.; Nittayananta, W.; Coogan, M.; Greenspan, D.; Greenspan, J.S. Periodontal disease in HIV/AIDS. Periodontology 2000 2012, 60, 78–97. [Google Scholar] [CrossRef]
- Woelber, J.P.; Tennert, C. Chapter 13: Diet and Periodontal Diseases. Monogr. Oral Sci. 2020, 28, 125–133. [Google Scholar] [CrossRef]
- Lee, D.E.; Won, S.Y. Relationship between clinical indicators of periodontal disease and serum level of Vitamin D. Curr. Res. Nutr. Food Sci. 2019, 7, 29–40. [Google Scholar] [CrossRef]
- Behura, S.S.; Rajesh, E.; Patra, A.; Aravindha, B.N. Nutrigenomics and its role in periodontal health—A review. Indian J. Forensic Med. Toxicol. 2020, 14, 1408–1411. [Google Scholar] [CrossRef]
- Bharti, V.; Bansal, C. Drug-induced gingival overgrowth: The nemesis of gingiva unravelled. J. Indian Soc. Periodontol. 2013, 17, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Heasman, P.A.; Hughes, F.J. Drugs, medications and periodontal disease. Br. Dent. J. 2014, 217, 411–419. [Google Scholar] [CrossRef] [Green Version]
- da Silva, M.K.; de Carvalho, A.; Alves, E.; da Silva, F.; Pessoa, L.; Vasconcelos, D. Genetic Factors and the Risk of Periodontitis Development: Findings from a Systematic Review Composed of 13 Studies of Meta-Analysis with 71,531 Participants. Int. J. Dent. 2017, 2017, 1914073. [Google Scholar] [CrossRef]
- Kumar, J.; Teoh, S.L.; Das, S.; Mahakknaukrauh, P. Oxidative Stress in Oral Diseases: Understanding Its Relation with Other Systemic Diseases. Front. Physiol. 2017, 8, 693. [Google Scholar] [CrossRef]
- Iacopino, A.M. Periodontitis and diabetes interrelationships: Role of inflammation. Ann. Periodontol. 2001, 6, 125–137. [Google Scholar] [CrossRef]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef]
- Griffin, S.O.; Jones, J.A.; Brunson, D.; Griffin, P.M.; Bailey, W.D. Burden of oral disease among older adults and implications for public health priorities. Am. J. Public Health 2012, 102, 411–418. [Google Scholar] [CrossRef]
- Ide, R.; Hoshuyama, T.; Wilson, D.; Takahashi, K.; Higashi, T. Periodontal disease and incident diabetes: A seven-year study. J. Dent. Res. 2011, 90, 41–46. [Google Scholar] [CrossRef]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J. Clin. Periodontol. 2018, 45, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontology 2000 2021, 87, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Kawaharada, R. Advanced Glycation End Products and Oxidative Stress in a Hyperglycaemic Environment. In Fundamentals of Glycosylation; Raghav, A., Ahmad, J., Eds.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Borgnakke, W.S. IDF Diabetes Atlas: Diabetes and oral health—A two-way relationship of clinical importance. Diabetes Res. Clin. Pract. 2019, 157, 107839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutler, C.W.; Machen, R.L.; Jotwani, R.; Iacopino, A.M. Heightened gingival inflammation and attachment loss in type 2 diabetics with hyperlipidemia. J. Periodontol. 1999, 70, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Bridges, R.B.; Anderson, J.W.; Saxe, S.R.; Gregory, K.; Bridges, S.R. Periodontal status of diabetic and non-diabetic men: Effects of smoking, glycemic control, and socioeconomic factors. J. Periodontol. 1996, 67, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.W.; Burt, B.A.; Becker, M.P.; Genco, R.J.; Shlossman, M.; Knowler, W.C.; Pettitt, D.J. Severe Periodontitis and Risk for Poor Glycemic Control in Patients with Non-Insulin-Dependent Diabetes Mellitus. J. Periodontol. 1996, 67 (Suppl. 10S), 1085–1093. [Google Scholar] [CrossRef] [Green Version]
- López, N.J.; Quintero, A.; Casanova, P.A.; Martínez, B. Routine prophylaxes every 3 months improves chronic periodontitis status in type 2 diabetes. J. Periodontol. 2014, 85, e232–e240. [Google Scholar] [CrossRef]
- Casanova, L.; Hughes, F.J.; Preshaw, P.M. Diabetes and periodontal disease: A two-way relationship. Br. Dent. J. 2014, 217, 433–437. [Google Scholar] [CrossRef] [Green Version]
- D’Aiuto, F.; Gkranias, N.; Bhowruth, D.; Khan, T.; Orlandi, M.; Suvan, J.; Masi, S.; Tsakos, G.; Hurel, S.; Hingorani, A.D.; et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: A 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. 2018, 6, 954–965. [Google Scholar] [CrossRef]
- Sczepanik, F.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontology 2000 2020, 84, 45–68. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S162–S170. [Google Scholar] [CrossRef] [PubMed]
- Oberti, L.; Gabrione, F.; Nardone, M.; Di Girolamo, M. Two-way relationship between diabetes and periodontal disease: A reality or a paradigm? J. Biol. Regul. Homeost. Agents 2019, 33 (Suppl. 1), 153–159. [Google Scholar]
- Polak, D.; Shapira, L. An update on the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J. Clin. Periodontol. 2018, 45, 150–166. [Google Scholar] [CrossRef]
- Lamster, I.B.; Lalla, E. Periodontal disease and diabetes mellitus: Discussion, conclusions, and recommendations. Ann. Periodontol. 2001, 6, 146–149. [Google Scholar] [CrossRef]
- Ilea, A.; Băbţan, A.M.; Boşca, B.A.; Crişan, M.; Petrescu, N.B.; Collino, M.; Sainz, R.M.; Gerlach, J.Q.; Câmpian, R.S. Advanced glycation end products (AGEs) in oral pathology. Arch. Oral Biol. 2018, 93, 22–30. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Yang, Y.; Zhang, X. An update on the potential role of advanced glycation end products in glycolipid metabolism. Life Sci. 2020, 245, 117344. [Google Scholar] [CrossRef]
- Gurav, A.N. Advanced glycation end products: A link between periodontitis and diabetes mellitus? Curr. Diabetes Rev. 2013, 9, 355–361. [Google Scholar] [CrossRef]
- Lalla, E.; Lamster, I.B.; Drury, S.; Fu, C.; Schmidt, A.M. Hyperglycemia, glycoxidation and receptor for advanced glycation endproducts: Potential mechanisms underlying diabetic complications, including diabetes-associated periodontitis. Periodontology 2000 2000, 23, 50–62. [Google Scholar] [CrossRef]
- Detzen, L.; Cheng, B.; Chen, C.Y.; Papapanou, P.N.; Lalla, E. Soluble Forms of the Receptor for Advanced Glycation Endproducts (RAGE) in Periodontitis. Sci. Rep. 2019, 9, 8170. [Google Scholar] [CrossRef] [PubMed]
- Hussein, L.K.; Mohammed, A.N. Assessment of serum advanced glycation end-product level and its effect on periodontal health status in type 2 diabetic patients with chronic periodontitis. Indian J. Forensic Med. Toxicol. 2020, 14, 609–615. [Google Scholar] [CrossRef]
- Yilmaz, D.; Topcu, A.O.; Akcay, E.U.; Altındis, M.; Gursoy, U.K. Salivary human beta-defensins and cathelicidin levels in relation to periodontitis and type 2 diabetes mellitus. Acta Odontol. Scand. 2020, 78, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Maiden, M.F.; Pham, C.; Kashket, S. Glucose toxicity effect and accumulation of methylglyoxal by the periodontal anaerobe Bacteroides forsythus. Anaerobe 2004, 10, 27–32. [Google Scholar] [CrossRef]
- Abbass, M.M.; Korany, N.S.; Salama, A.H.; Dmytryk, J.J.; Safiejko-Mroczka, B. The relationship between receptor for advanced glycation end products expression and the severity of periodontal disease in the gingiva of diabetic and non diabetic periodontitis patients. Arch. Oral Biol. 2012, 57, 1342–1354. [Google Scholar] [CrossRef]
- Katz, J.; Bhattacharyya, I.; Farkhondeh-Kish, F.; Perez, F.M.; Caudle, R.M.; Heft, M.W. Expression of the receptor of advanced glycation end products in gingival tissues of type 2 diabetes patients with chronic periodontal disease: A study utilizing immunohistochemistry and RT-PCR. J. Clin. Periodontol. 2005, 32, 40–44. [Google Scholar] [CrossRef]
- Zizzi, A.; Tirabassi, G.; Aspriello, S.D.; Piemontese, M.; Rubini, C.; Lucarini, G. Gingival advanced glycation end-products in diabetes mellitus-associated chronic periodontitis: An immunohistochemical study. J. Periodontal Res. 2013, 48, 293–301. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Y.; Ye, C.; Wu, W.; Liao, G.; Lu, Y.; Huang, P. EB 2017 Article: Changes in advanced glycation end products, beta-defensin-3, and interleukin-17 during diabetic periodontitis development in rhesus monkeys. Exp. Biol. Med. 2018, 243, 684–694. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [Green Version]
- Settem, R.P.; Honma, K.; Shankar, M.; Li, M.; LaMonte, M.; Xu, D.; Genco, R.J.; Browne, R.W.; Sharma, A. Tannerella forsythia-produced methylglyoxal causes accumulation of advanced glycation endproducts to trigger cytokine secretion in human monocytes. Mol. Oral Microbiol. 2018, 33, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Al-Sowygh, Z.H.; Ghani, S.; Sergis, K.; Vohra, F.; Akram, Z. Peri-implant conditions and levels of advanced glycation end products among patients with different glycemic control. Clin. Implant. Dent. Relat. Res. 2018, 20, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Bhawal, U.K.; Sasahira, T.; Toyama, T.; Sato, T.; Matsuda, D.; Nishikiori, H.; Kobayashi, M.; Sugiyama, M.; Hamada, N.; et al. Involvement of HMGB1 and RAGE in IL-1β-induced gingival inflammation. Arch. Oral Biol. 2012, 57, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Celecová, V.; Kamodyová, N.; Tóthová, L.; Kúdela, M.; Celec, P. Salivary markers of oxidative stress are related to age and oral health in adult non-smokers. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2013, 42, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Ojima, M.; Yoshioka, H.; Inaba, H.; Kogo, M.; Shizukuishi, S.; Nomura, M.; Amano, A. Relationship of serum advanced glycation end products with deterioration of periodontitis in type 2 diabetes patients. J. Periodontol. 2006, 77, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.L.; Tsai, C.C.; Wang, Y.Y.; Ho, K.Y.; Wu, Y.M.; Hung, H.C.; Lin, Y.C. The association between the RAGE G82S polymorphism, sRAGE and chronic periodontitis in Taiwanese individuals with and without diabetes. J. Periodontal Res. 2015, 50, 881–889. [Google Scholar] [CrossRef]
- Yu, S.; Li, H.; Ma, Y.; Fu, Y. Matrix metalloproteinase-1 of gingival fibroblasts influenced by advanced glycation end products (AGEs) and their association with receptor for AGEs and nuclear factor-κB in gingival connective tissue. J. Periodontol. 2012, 83, 119–126. [Google Scholar] [CrossRef]
- Grimm, W.D.; Shchetinin, E.V.; Bobrishev, D.V.; Sirak, S.V. Quantifying analysis of advanced glycosylation end products (ages) expression in periodontitis patients with diabetes type II. Med. News N. Cauc. 2015, 10, 178–183. [Google Scholar]
- Yoon, M.S.; Jankowski, V.; Montag, S.; Zidek, W.; Henning, L.; Schlüter, H.; Tepel, M.; Jankowski, J. Characterisation of advanced glycation endproducts in saliva from patients with diabetes mellitus. Biochem. Biophys. Res. Commun. 2004, 323, 377–381. [Google Scholar] [CrossRef]
- Kashket, S.; Maiden, M.F.; Haffajee, A.D.; Kashket, E.R. Accumulation of methylglyoxal in the gingival crevicular fluid of chronic periodontitis patients. J. Clin. Periodontol. 2003, 30, 364–367. [Google Scholar] [CrossRef]
- Akram, Z.; Alqahtani, F.; Alqahtani, M.; Al-Kheraif, A.A.; Javed, F. Levels of advanced glycation end products in gingival crevicular fluid of chronic periodontitis patients with and without type-2 diabetes mellitus. J. Periodontol. 2020, 91, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Rajeev, K.; Karthika, R.; Mythili, R.; Krishnan, V.; Nirmal, M. Role of receptors of advanced glycation end-products (RAGE) in type 2 diabetic and non-diabetic individuals with chronic periodontal disease: An immunohistochemical study. J. Investig. Clin. Dent. 2011, 2, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Pradeep, A.R.; Kanoriya, D.; Garg, V. Human soluble receptor for advanced glycation end products and tumor necrosis factor-α as gingival crevicular fluid and serum markers of inflammation in chronic periodontitis and type 2 diabetes. J. Oral Sci. 2016, 58, 547–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, P.C.; Chien, L.Y.; Yeo, J.F.; Wang, Y.P.; Chung, M.C.; Chong, L.Y.; Kuo, M.Y.; Chen, C.H.; Chiang, H.C.; Ng, B.N.; et al. Progression of periodontal destruction and the roles of advanced glycation end products in experimental diabetes. J. Periodontol. 2013, 84, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Akshata, K.R.; Ranganath, V.; Nichani, A.S. Thesis, antithesis, and synthesis in periodontal and systemic interlink. J. Indian Soc. Periodontol. 2012, 16, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Choromańska, M.; Klimiuk, A.; Kostecka-Sochoń, P.; Wilczyńska, K.; Kwiatkowski, M.; Okuniewska, N.; Waszkiewicz, N.; Zalewska, A.; Maciejczyk, M. Antioxidant Defence, Oxidative Stress and Oxidative Damage in Saliva, Plasma and Erythrocytes of Dementia Patients. Can Salivary AGE be a Marker of Dementia? Int. J. Mol. Sci. 2017, 18, 2205. [Google Scholar] [CrossRef] [Green Version]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Wright, E., Jr.; Scism-Bacon, J.L.; Glass, L.C. Oxidative stress in type 2 diabetes: The role of fasting and postprandial glycaemia. Int. J. Clin. Pract. 2006, 60, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Patil, V.S.; Patil, V.P.; Gokhale, N.; Acharya, A.; Kangokar, P. Chronic Periodontitis in Type 2 Diabetes Mellitus: Oxidative Stress as a Common Factor in Periodontal Tissue Injury. J. Clin. Diagn. Res. JCDR 2016, 10, BC12–BC16. [Google Scholar] [CrossRef]
- Andriankaja, O.M.; Barros, S.P.; Moss, K.; Panagakos, F.S.; DeVizio, W.; Beck, J.; Offenbacher, S. Levels of serum interleukin (IL)-6 and gingival crevicular fluid of IL-1beta and prostaglandin E(2) among non-smoking subjects with gingivitis and type 2 diabetes. J. Periodontol. 2009, 80, 307–316. [Google Scholar] [CrossRef]
- Tan, K.C.; Chow, W.S.; Tam, S.; Bucala, R.; Betteridge, J. Association between acute-phase reactants and advanced glycation end products in type 2 diabetes. Diabetes Care 2004, 27, 223–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001, 286, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Xiao, E.; Mattos, M.; Vieira, G.; Chen, S.; Corrêa, J.D.; Wu, Y.; Albiero, M.L.; Bittinger, K.; Graves, D.T. Diabetes Enhances IL-17 Expression and Alters the Oral Microbiome to Increase Its Pathogenicity. Cell Host Microbe 2017, 22, 120–128.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Kardeşler, L.; Buduneli, N.; Biyikoğlu, B.; Cetinkalp, S.; Kütükçüler, N. Gingival crevicular fluid PGE2, IL-1beta, t-PA, PAI-2 levels in type 2 diabetes and relationship with periodontal disease. Clin. Biochem. 2008, 41, 863–868. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollreisz, A.; Hudson, B.I.; Chang, J.S.; Qu, W.; Cheng, B.; Papapanou, P.N.; Schmidt, A.M.; Lalla, E. Receptor for advanced glycation endproducts mediates pro-atherogenic responses to periodontal infection in vascular endothelial cells. Atherosclerosis 2010, 212, 451–456. [Google Scholar] [CrossRef] [Green Version]
- Soory, M. Redox status in periodontal and systemic inflammatory conditions including associated neoplasias: Antioxidants as adjunctive therapy? Infect. Disord. Drug Targets 2009, 9, 415–427. [Google Scholar] [CrossRef] [Green Version]
- Stern, D.; Yan, S.D.; Yan, S.F.; Schmidt, A.M. Receptor for advanced glycation endproducts: A multiligand receptor magnifying cell stress in diverse pathologic settings. Adv. Drug Deliv. Rev. 2002, 54, 1615–1625. [Google Scholar] [CrossRef]
- Dietrich, I.; Braga, G.A.; de Melo, F.G.; da Costa Silva Silva, A. The Diabetic Foot as a Proxy for Cardiovascular Events and Mortality Review. Curr. Atheroscler. Rep. 2017, 19, 44. [Google Scholar] [CrossRef]
- Yamagishi, S.; Maeda, S.; Matsui, T.; Ueda, S.; Fukami, K.; Okuda, S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochim. Biophys. Acta 2012, 1820, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko, I.V.; Furalyov, V.A.; Popov, V.O. Glycated albumin stimulates expression of inflammatory cytokines in muscle cells. Cytokine 2020, 128, 154991. [Google Scholar] [CrossRef] [PubMed]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes mellitus and inflammation. Curr. Diabetes Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.V.; Shaw, L.C.; Grant, M.B. Inflammation in the pathogenesis of microvascular complications in diabetes. Front. Endocrinol. 2012, 3, 170. [Google Scholar] [CrossRef] [Green Version]
- Zephy, D.; Ahmad, J. Type 2 diabetes mellitus: Role of melatonin and oxidative stress. Diabetes Metab. Syndr. 2015, 9, 127–131. [Google Scholar] [CrossRef]
- Luo, S.; Yang, X.; Wang, D.; Ni, J.; Wu, J.; Xu, Z.; Xuan, D.; Zhang, J. Periodontitis contributes to aberrant metabolism in type 2 diabetes mellitus rats by stimulating the expression of adipokines. J. Periodontal Res. 2016, 51, 453–461. [Google Scholar] [CrossRef]
- Evans, J.L.; Maddux, B.A.; Goldfine, I.D. The molecular basis for oxidative stress-induced insulin resistance. Antioxid. Redox Signal. 2005, 7, 1040–1052. [Google Scholar] [CrossRef]
- Taylor, J.J.; Preshaw, P.M.; Lalla, E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J. Periodontol. 2013, 84 (Suppl. 4), S113–S134. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Zhang, W.; Liu, X.; Zhang, W.; Li, Y. Interrelationship between diabetes and periodontitis: Role of hyperlipidemia. Arch. Oral Biol. 2015, 60, 667–674. [Google Scholar] [CrossRef]
- Ogawa, T.; Asai, Y.; Hashimoto, M.; Takeuchi, O.; Kurita, T.; Yoshikai, Y.; Miyake, K.; Akira, S. Cell activation by Porphyromonas gingivalis lipid A molecule through Toll-like receptor 4- and myeloid differentiation factor 88-dependent signaling pathway. Int. Immunol. 2002, 14, 1325–1332. [Google Scholar] [CrossRef]
- Streja, D.; Cressey, P.; Rabkin, S.W. Associations between inflammatory markers, traditional risk factors, and complications in patients with type 2 diabetes mellitus. J. Diabetes Its Complicat. 2003, 17, 120–127. [Google Scholar] [CrossRef]
- Tian, J.; Liu, C.; Zheng, X.; Jia, X.; Peng, X.; Yang, R.; Zhou, X.; Xu, X. Porphyromonas gingivalis Induces Insulin Resistance by Increasing BCAA Levels in Mice. J. Dent. Res. 2020, 99, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.H.; Jan, A.T.; Rabbani, G.; Ahmad, K.; Ashraf, J.M.; Kim, T.; Min, H.S.; Lee, Y.H.; Cho, W.K.; Ma, J.Y.; et al. Methylglyoxal and Advanced Glycation End products: Insight of the regulatory machinery affecting the myogenic program and of its modulation by natural compounds. Sci. Rep. 2017, 7, 5916. [Google Scholar] [CrossRef] [PubMed]
- Borgnakke, W.S.; Ylöstalo, P.V.; Taylor, G.W.; Genco, R.J. Effect of periodontal disease on diabetes: Systematic review of epidemiologic observational evidence. J. Periodontol. 2013, 84 (Suppl. 4), S135–S152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winning, L.; Patterson, C.C.; Neville, C.E.; Kee, F.; Linden, G.J. Periodontitis and incident type 2 diabetes: A prospective cohort study. J. Clin. Periodontol. 2017, 44, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Elkind-Hirsch, K.E.; Xie, Y.; Delarosa, R.; Maney, P.; Pridjian, G.; Buekens, P. Periodontal disease as a potential risk factor for the development of diabetes in women with a prior history of gestational diabetes mellitus. J. Public Health Dent. 2013, 73, 41–49. [Google Scholar] [CrossRef]
- Falcao, A.; Bullón, P. A review of the influence of periodontal treatment in systemic diseases. Periodontology 2000 2019, 79, 117–128. [Google Scholar] [CrossRef]
- Arai, K.; Nishikawa, T.; Matsuzawa, Y.; Ohtsu, S.; Shirabe, S.I.; Yuasa, S.; Hirao, K.; Mori, H. Differences in Dental Care Referral for Diabetic Patients Between General Practitioners and Diabetes Specialists in Japan, Analyzed from NSAID-Study 3. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2022, 13, 379–385. [Google Scholar] [CrossRef]
- Jain, A.; Gupta, J.; Bansal, D.; Sood, S.; Gupta, S.; Jain, A. Effect of scaling and root planing as monotherapy on glycemic control in patients of Type 2 diabetes with chronic periodontitis: A systematic review and meta-analysis. J. Indian Soc. Periodontol. 2019, 23, 303–310. [Google Scholar] [CrossRef]
- Baeza, M.; Morales, A.; Cisterna, C.; Cavalla, F.; Jara, G.; Isamitt, Y.; Pino, P.; Gamonal, J. Effect of periodontal treatment in patients with periodontitis and diabetes: Systematic review and meta-analysis. J. Appl. Oral Sci. Rev. FOB 2020, 28, e20190248. [Google Scholar] [CrossRef]
- Marconcini, S.; Giammarinaro, E.; Cosola, S.; Oldoini, G.; Genovesi, A.; Covani, U. Effects of Non-Surgical Periodontal Treatment on Reactive Oxygen Metabolites and Glycemic Control in Diabetic Patients with Chronic Periodontitis. Antioxidants 2021, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Lovati, E.; Rizzotto, S.; Segù, M.; Scribante, A.; Lanteri, V.; Chiesa, A.; Granata, M.; Ruggero, R.Y.B. Professional and Home-Management in non -surgical periodontal therapy to evaluate the percentage of glycated hemoglobin in type 1 diabetes patients. Int. J. Clin. Dent. 2021, 14, 41–53. [Google Scholar]
- Mizuno, H.; Ekuni, D.; Maruyama, T.; Kataoka, K.; Yoneda, T.; Fukuhara, D.; Sugiura, Y.; Tomofuji, T.; Wada, J.; Morita, M. The effects of non-surgical periodontal treatment on glycemic control, oxidative stress balance and quality of life in patients with type 2 diabetes: A randomized clinical trial. PLoS ONE 2017, 12, e0188171. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| S/No | Database | Search String Used | Total Number of Articles |
|---|---|---|---|
| 1. | PubMed | [“periodontitis” OR “periodontal disease” OR “Chronic periodontitis” OR “periodontal Inflammation”) AND (“diabetes” OR “diabetes mellitus” OR “Type 2 DM” OR “Type 2 diabetes mellitus” OR “non-insulin dependent diabetes mellitus” OR “Hyperglycemia” OR Insulin resistance)) AND (“Advanced glycation end products” OR “Advanced glycosylation end products” OR “Millard Reaction” OR “carboxymethylysine” OR “Pentosidine”) | 119 |
| 2. | Scopus | “Periodontitis” OR “Periodontal disease” OR “Chronic periodontitis” OR “Periodontal Inflammation”) AND (“Diabetes” OR “Diabetes mellitus” OR “Type 2 DM” OR “Type 2 diabetes mellitus” OR “non-insulin-dependent diabetes mellitus” OR “Hyperglycemia” OR “Insulin resistance”) AND (“Advanced glycation end product” OR “Advanced glycosylation end products” OR “Millard Reaction” OR “carboxymethylysine” OR “Pentosidine”) | 958 |
| 3. | Web of Science | TI = (“Periodontitis” OR “periodontal disease” OR “Chronic periodontitis” OR “periodontal Inflammation”) AND (“diabetes” OR “diabetes mellitus” OR “Type 2 DM” OR “Type 2 diabetes mellitus” OR “non-insulin dependent diabetes mellitus” OR “Hyperglycemia” OR Insulin resistance)) AND (“Advanced glycation end products” OR “Advanced glycosylation end products” OR “Millard Reaction” OR “carboxymethylysine” OR “Pentosidine”) | 6 |
| 4. | Dentistry and Open Science (EBSCO) | TI (Periodontitis OR periodontal disease OR Chronic periodontitis OR periodontal Inflammation AND Diabetes OR diabetes mellitus OR Type 2 DM OR Type 2 diabetes mellitus OR non-insulin dependent diabetes mellitus OR Hyperglycemia OR Insulin resistance AND Advanced glycation end products OR Advanced glycosylation end products OR Millard Reaction OR carboxymethylysine OR Pentosidine) | 5 |
| 5. | Cochrane Database | Periodontitis OR periodontal disease OR Chronic periodontitis OR periodontal Inflammation in Title Abstract Keyword AND Diabetes OR diabetes mellitus OR Type 2 DM OR Type 2 diabetes mellitus OR non-insulin dependent diabetes mellitus OR Hyperglycemia OR Insulin resistance in Title Abstract Keyword AND Advanced glycation end products OR Advanced glycosylation end products OR Millard Reaction OR carboxymethylysine OR Pentosidine in Title Abstract Keyword—with Publication Year from 2000 to 2020, in Trials | 6 |
| 6. | Clinicaltrials.gov | Periodontitis and advanced glycation End products | 46 |
| Total search | 1140 |
| Author/Year/Country of Origin/Study Design | Male/Female Ratio, AGE Range or Mean Age | Diagnostic Criteria for Periodontitis | Diagnostic Criteria for Diabetes | Method Used to Analyze AGE or Its Receptors | Groups in the Study and Sample Size in Each Group (n) | Outcome Analyzed Mean PD, CAL, BOP, and AGE Levels | ||||
| AGEs/RAGE Levels | Any Forms of RAGE to Its Receptor | PD (in mm) | CAL (in mm) | BOP (%) | ||||||
| Serum | ||||||||||
| Takeda et al. (2006) [66] Japan Analytical cross-sectional | Male:Female: 1.2:1.0. Age range: 20–35 years (mean age: 57.8–12.1 years) | Presence or absence of BOP with CP: CAL > 5 mm. Periodontal health: CAL in a tooth in <5 mm | World Health Organization (not specified) | ELISA | DM + PD n = 69 | 2.5 ± 0.8 mU/mL | - | - | - | 3.3 ± 4.8 |
| DM + no PD n = 28 | 2.6 ± 1.0 mU/mL | - | - | - | 1.4 ± 2.8 | |||||
| Hussein and Mohammed (2020) [53] Iraq Analytical cross-sectional | Only male patients. Age range: 35–55 years | Periodontitis: CAL ≥ 5 mm Periodontal health: no CAL | HbA1c > 9% | ELISA | DM + PD n = 30 | 26.92 ng/mL | - | 5.58 ± 0.48 | 5.57 ± 0.55 | 73.01 ± 14.60 |
| No DM + PD n = 30 | 15.91 ng/mL | - | 5.24 ± 0.64 | 5.14 ± 0.41 | 70.40 ± 9.27 | |||||
| No DM + No PD n = 20 | 6.60 ng/mL | - | - | - | - | |||||
| Detzen et al. (2019) [52] USA Case-control study | Only male patients (100 males in each group). Mean age: 42.9 ± 9.9 years |
Periodontitis group: ≥5 mm PD along with CAL ≥ 3 mm, and BOP ≥ 30% of their surfaces. Periodontal healthy: no sites with ≥5 mm PD and CAL attachment loss of < 3 mm | Normoglycemic patients | ELISA/Immunohistochemistry/spectrophotometry | No DM + PD n = 50 | sRAGE levels = 0.95 ± 0.43 ng/mL esRAGE: 0.29 ± 0.15 ng/mL cRAGE: 0.66 ± 0.31 ng/mL | 4.5-fold increase in the expression of cRAGE and 2.3 folds increase in AGER1 expression in periodontitis-affected sites compared to control | 3.0 ± 1.0 | 2.3 ± 1.6 | 37.7 ± 26.6 |
| No DM + No PD n = 50 | sRAGE = 1.17 ± 0.40 ng/mL esRAGE: 0.30 ± 0.12 ng/mL cRAGE: 0.88 ± 0.79 ng/mL | - | - | - | ||||||
| Wu et al. (2015) [71] Taiwan Case control study | Male: Female: DM + PD: 1:1 DM + no PD: 1:1 No DM + PD: 7.7:1.0 No DM + No PD: 1.8:1.0 Mean age: 42.1 ± 6.4 to 44.7 ± 6.1 years | Periodontitis group: CPI score of 0, 1 or 2: Non periodontitis group Periodontal healthy: CPI score 3 or 4: CP group | Individuals with DM and healthy controls | ELISA | DM + PD n = 172 | sRAGE with the G82G genotype in plasma: 889.5 ± 493.3 | - | - | - | - |
| DM + NO PD n = 58 | sRAGE in plasma with the G82G genotype: 991.3 ± 507.6 pg/mL | - | - | - | - | |||||
| No DM + PD n = 202 | sRAGE in plasma with the G82G genotype: 845.1 ± 335.0 pg/mL | - | - | - | - | |||||
| No DM + No PD n = 62 | sRAGE in plasma with the G82G genotype: 890.5 ± 326.4 pg/mL | - | - | - | ||||||
| Serum and Gingival Crevicular Fluid | ||||||||||
| Singhal et al., 2016 [74] India Analytical cross-sectional | Male:Female: 1:1 Age range: 25–42 years | Periodontitis group: CAL ≥ 3 mm, GI > 1, PD ≥ 5 mm, and radiographic bone loss. Periodontal health: PD ≤ 3 mm, GI = 0, CAL = 0, and HbA1c ≤ 7%) and no radiographic evidence of bone loss | DM = HbA1c ≤ 7% (minimum 5 years of DM) | ELISA | DM + PD n = 20 | - | sRAGE (GCF): 442.425 ± 72.88 pg/mL sRAGE (Serum): 460.23 ± 81.23 pg/ml | 2 ± 0.655 | 5.60 ± 0.88 | - |
| DM + no PD n = 15 | - | sRAGE (GCF): 536.88 ± 78.83 pg/mL sRAGE (serum): 555.99 ± 83.53 pg/ml | 6.60 ± 1.27 | 0 | - | |||||
| No DM + PD n = 20 | - | sRAGE (GCF): (607.56 ± 84.40 pg/mL sRAGE (serum): 626.565 ± 84.54 pg/mL | 6.15 ± 1.09 | 5.05 ± 1.67 | - | |||||
| No DM + No PD n = 15 | - | sRAGE (GCF): 690.74 ± 68.38 pg/mL sRAGE (serum) 732.88 ± 68.97 pg/mL | 2 ± 0.66 | 0 | - | |||||
| Akram et al. (2020) [72] Kingdom of Saudi Arabia Analytical cross-sectional | DM + PD = Male:Female: 5.4:1.0 Mean age: 55.2 yearsNo DM + PD = Male:Female: 4.1:1.0 Mean Age: 51.5 years No DM + No PD: Male/Female:6.75:1.0 Mean age: 50.7 years | Periodontitis group: Presence of BOP, Pl, PD ≥ 4 mm, CAL ≥ 3 mm, and MBL ≥ 3 mm at six sites per tooth at least 30% of sites. Periodontal health: not mentioned | Normoglycemic→ HbA1c levels of ≤5.6% Hyperglycemic → HbA1c levels of ≥ 5.6% | GCF | DM + PD n = 32 | 521.9 pg/mL | - | 49.2% (p < 0.001) | - | 5.2% (3.5 to 5.8%) (p < 0.001) |
| No DM + PD n = 31 | 234.84 pg/mL | - | 34.4% (p < 0.01) | - | 3.3% (3 to 5%) | |||||
| No DM + No PD n = 31 | 87.2 pg/mL | - | 0% (p < 0.001) | - | 0.8% (0 to 1.5%) | |||||
| Kashket et al., (2003) [71] USA Analytical cross sectional | Male: Female ratio: No PD: 2.5:1.0 Mean Age: Healthy: 45.67 ± 5.7 years Male:Female ratio in PD group: 0.4:1.0 Mean age: 45.37 ± 13.1 years | Periodontitis group: minimum of four teeth with pocket depths or attachment loss of 4 mm | Normoglycemic patient (not mentioned about the glycemic control) | GCF | PD with disease sites n = 14 | MG levels: 200.7 ± 243.1 pmol/sites | - | Mean PD with sites from which MG was collected: 5.77 ± 0.7 mm | 3.27 ± 0.8 mm | 24 ± 13 |
| PD with Healthy sites n = 14 | MG levels: 140.9 ± 236.6 picomoles/sites | - | Whole mouth PD with sites from which MG was collected: 2.77 ± 0.6 mm | 3.27 ± 0.8 mm | ||||||
| Healthy controls n = 7 | Mean MG levels: 11.5 ± 4.4 | - | Mean PD with sites from which was collected: 2.77 ± 0.5 mm | 2.07 ± 0.4 | 10 ± 10 | |||||
| Gingival Tissues | ||||||||||
| Katz et al., 2005 [57] Analytical cross-sectional | Male:female: Not mentioned Mean age or age range: Not mentioned | Generalized periodontal disease: CAL 30% with BOP Patients with active periodontal inflammation Periodontal health: not mentioned | Fasting blood glucose levels of <126 mg/dl as reported by the patient for without type 2 diabetes mellitus and patients with diabetes mellitus > 126 mg/dL | Immunohistochemistry, m-RNA expression and PCR | DM + PD n = 8 | The RAGE increased in the spinous and basal layer of the inflamed gingival epithelium in subjects with and without T2DM having periodontitis. Approximately 50% increase in RAGE mRNA was noted in the gingiva of patients with DM with periodontitis compared having healthy controls with periodontitis (p < 0.05). | ||||
| No DM + PD n = 14 | ||||||||||
| Rajeev et al., 2011 India [73] Analytical cross-sectional | Male: Female: Not mentioned Mean age: not mentioned | Periodontal disease consisting of a PD of ≥ 3 mm and CAL of ≥6 mm Periodontal health: not mentioned | World Health Organization (b) moderately controlled patients with diabetes mellitus and 6–8% HbA1c | Immunohistochemistry | DM + PD n = 19 | All groups showed mild to strong immunoreactivity for RAGE receptor. The patients with T2DM and chronic periodontitis had more BOP, PD, and CAL compared to the control group (p < 0.01). A positive correlation between RAGE receptor activation, age and HbA1c were noted. | ||||
| No DM + PD n = 18 | Six patients showed mild immune reactivity for RAGE. Twelve showed negative immune reactivity for RAGE. | |||||||||
| Abbass et al., 2012 [56] U.S.A)(break/Analytical cross-sectional | Male: Female: 1.2: 1.0 Mean age: 53–60 years | Periodontitis: presence of at minimum 5 sites with ≥4 mm; horizontal alveolar bone loss noted in the radiographs. PD and CAL | DM: HbA1c ≥ 6.5%No DM: HbA1c (4% to 5.9%) | Immunohistochemistry | DM + PD n = 15 | - | 46.91 b ± 5.57 | 6.8 ± 1.146 | 7.8 ± 1.424 | - |
| No DM + PD n = 25 | 31.42 c ± 7.42 | 5.8 ± 1.832 | 6.6 ± 1.917 | - | ||||||
| No DM + No PD n = 10 | - | 21.54 ± 1.46 | 5.8 ± 1.832 | 6.6 ± 1.917 | ||||||
| Zizzi et al., 2013 [58] Italy Analytical cross-sectional | DM + PD group: Male:female: 4.33:1.0 Mean age: 59 ± 1.25 years No DM + PD: Male/female: 1.28: 1.00 Mean age: 56.5 ± 1.32 yearsNo DM + No PD = Male/female:3:1 Mean Age = 55 ± 1.76 years | Generalized, severe, chronic periodontitis: more than 30% of the sites with > 5 mm CAL. Periodontal Health: PD < 3 mm, gingival index = 0 (absence of clinical inflammation) and CAL < 2 mm | Diabetes patients → No diabetes mellitus: glycated hemoglobin (HbA1c) < 6.1% and plasma glycemia lower than 100 mg/dL | Immunohistochemistry | DM + PD n = 16 | - | Epithelium (AGE%) = 75 (65–80) Vessels (AGE%) = 63.4 ± 1.97 | 7 (7–7.2) | 6.4 (6.3–6.6) | 2 (1.4–3) |
| No DM + PD n = 16 | - | Epithelium (AGE%) =70 Vessels (AGE%) =58.7 ± 4.19 | 7.1 | 6.6 | 1.9 | |||||
| No DM + No PD n = 16 | - | Epithelium (AGE%) = 62.5 Vessels (AGE%) = 51.8 ± 2.88 | 2.6 | 1.1 | - | |||||
| (Grimm et al., 2015) [69] Russia and Germany Cross-sectional study | Male/female ratio: 1.22: 1.00 Age range: 40- 68 years | Periodontitis: >30% of the sites: PD > 4 mm | Not mentioned | Immunohistochemistry | DM + PD n = 10 | Immunohistochemistry showed a 1.3-fold increase in the percentage of AGE deposition in subjects with DM (17%) compared to those without DM (13%). AGE deposition more in the vascular structures, epithelial and connective tissues’ cells. | ||||
| NO DM + PD n = 10 | ||||||||||
| Saliva | ||||||||||
| Yilmaz et al., 2020 [54] Turkey Analytical cross-sectional | % of females in each group: No DM + No PD = 17.3; No DM + PD = 15.3; DM + No PD = 33.7; DM + PD = 33.7 | Periodontitis group: BOP ≥10% and interdental CAL at ≥2 non-adjacent teeth with PPD ≥ 4 mm. Periodontal health: BOP ˂ 10% of the surfaces and no sites with PPD > 3 mm besides no CAL or bone loss | Fasting plasma glucose (FPG) ≥126 mg/dL (7.0 mmol/L, and HbA1c ≥ 6.5% (48 mmol/mol) | ELISA | DM + PD n = 63 | AGE: 332 ± 350 ng/mL | - | 5.52 ± 0.53 | 6.29 ± 0.69 | - |
| DM + no PD n = 58 | AGE: 235 ± 360 ng/mL | - | 2.31 ± 0.37 | 2.59 ± 0.65 | - | |||||
| No DM + PD n = 29 | AGE levels: 46.8 ± 52.1 ng/mL | - | 5.30 ± 0.53 | 5.80 ± 0.72 | - | |||||
| No DM + No PD n = 28 | AGE levels: 24.4 ± 8.5 ng/mL | - | 2.26 ± 0.38 | 2.67 ± 0.69 | - | |||||
| S/No | Author, Year | Selection | Comparability | Outcomes | ROB Scores | ||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the Sample: | Sample Size | Non-Respondents | Ascertainment of the Exposure (Risk Factor): | Comparability of Subjects in Different Outcome Groups Based on Design or Analysis. Confounding Factors Controlled | Assessment of Outcome: | Statistical Test | |||
| 1. | Rajeev et al., 2011 [73] | b | b | b | a | b | B | c | 4 |
| 2. | Akram et al., 2020 [72] | a | b | a | a | a/b | a | a | 9 |
| 3. | Abbass et al., 2012 [56] | a | b | a | a | a | B | a | 7 |
| 4. | Takeda et al., 2006 [66] | a | b | a | a | a | b | a | 7 |
| 5. | Katz et al., 2005 [57] | a | b | b | b | a/b | b | a | 6 |
| 6. | Singhal et al., 2016 [74] | a | b | a | a | a/b | b | a | 6 |
| 7. | Yilmaz et al., 2020 [54] | a | a | b | b | a/b | a | a | 7 |
| 8. | Zizzi et al., 2013 [58] | a | b | a | b | a/b | b | a | 7 |
| 9. | Hussein and Mohammed (2020) [53] | a | b | a | a | b | b | a | 7 |
| 10. | Detzen et al., 2019 [52] | a | a | a | a | a/b | A | a | 9 |
| 11. | Kashket et al., 2003 [71] | a | a | a | a | b | a | a | 9 |
| 12. | Grimm et al., 2015 [69] | b | c | b | b | b | b | b | 4 |
| 13. | Wu et al., 2015 [67] | a | b | b | b | a | a | a | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chopra, A.; Jayasinghe, T.N.; Eberhard, J. Are Inflamed Periodontal Tissues Endogenous Source of Advanced Glycation End-Products (AGEs) in Individuals with and without Diabetes Mellitus? A Systematic Review. Biomolecules 2022, 12, 642. https://doi.org/10.3390/biom12050642
Chopra A, Jayasinghe TN, Eberhard J. Are Inflamed Periodontal Tissues Endogenous Source of Advanced Glycation End-Products (AGEs) in Individuals with and without Diabetes Mellitus? A Systematic Review. Biomolecules. 2022; 12(5):642. https://doi.org/10.3390/biom12050642
Chicago/Turabian StyleChopra, Aditi, Thilini N. Jayasinghe, and Joerg Eberhard. 2022. "Are Inflamed Periodontal Tissues Endogenous Source of Advanced Glycation End-Products (AGEs) in Individuals with and without Diabetes Mellitus? A Systematic Review" Biomolecules 12, no. 5: 642. https://doi.org/10.3390/biom12050642