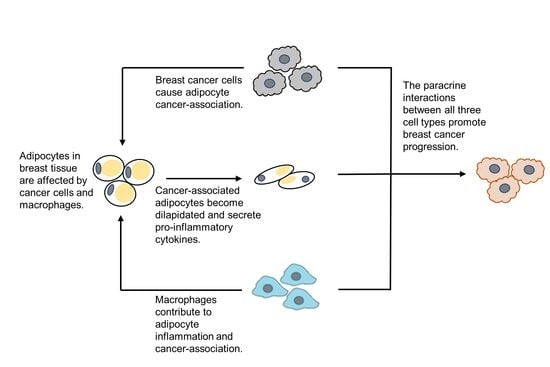
Macrophage-Conditioned Media Promotes Adipocyte Cancer Association, Which in Turn Stimulates Breast Cancer Proliferation and Migration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Differentiation of hMSCs into Adipocytes
2.3. Differentiation of U937 Cells into Macrophages
2.4. Macrophage-Conditioned Media (CM) Preparation
2.5. Breast Cancer–Adipocyte Co-Culture
2.6. Breast Cancer Cell Counts
2.7. Wound Healing Assay
2.8. Real-Time Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
2.9. Statistical Analysis
3. Results
3.1. Quantitative RT-PCR Analysis of U937 Monocyte-Derived Macrophages
3.2. Breast Cancer Cell Proliferation and Migration
3.3. Quantitative RT-PCR Analysis of Adipocytes
4. Discussion
4.1. Macrophage-Conditioned Media Has Varying Effectiveness upon Different Types of Breast Cancer Cells
4.2. Macrophage-Conditioned Media Increases the Pro-Inflammatory Character of Adipocytes
4.3. Macrophage-Conditioned Media Increases Matrix Metalloproteinase Expression in Adipocytes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-MEM | Minimum Essential Medium: Eagle Alpha Modification |
| AdipoQ | Adiponectin |
| BRCA | Breast cancer |
| CAA | Cancer-associated adipocytes |
| CD11b | Cluster of differentiation molecule 11B |
| CM | Conditioned media |
| ECM | Extracellular matrix |
| FBS | Fetal bovine serum |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| HMSCs | Human mesenchymal stem cells |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| LEP | Leptin |
| MMP-3 | Matrix metalloproteinase-3 |
| MMP-11 | Matrix metalloproteinase-11 |
| NonCM | Non-conditioned media |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PMA | Phorbol myristate acetate |
| RETN | Resistin |
| RQ | Relative quantification |
| TNF-α | Tumor necrosis factor-α |
References
- World Health Organization. Obesity and Overweight—Fact Sheet (Updated February 2018). Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 15 April 2019).
- Stierman, B.; Afful, J.; Carroll, M.D.; Chen, T.-C.; Davy, O.; Fink, S.; Fryar, C.D.; Gu, Q.; Hales, C.M.; Hughes, J.P.; et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021.
- Bhaskaran, K.; Douglas, I.; Forbes, H.; dos-Santos-Silva, I.; Leon, D.A.; Smeeth, L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5·24 million UK adults. Lancet 2014, 384, 755–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, C.K.; Wiggins, C.L.; Nibbe, A.M.; Storlie, C.B.; Prossnitz, E.R.; Royce, M.; Lomo, L.C.; Hill, D.A. Obesity and survival among a cohort of breast cancer patients is partially mediated by tumor characteristics. npj Breast Cancer 2019, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Ma, J. Body Mass Index, Prostate Cancer–Specific Mortality, and Biochemical Recurrence: A Systematic Review and Meta-analysis. Cancer Prev. Res. 2011, 4, 486–501. [Google Scholar] [CrossRef] [Green Version]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; Le Gonidec, S.; et al. Cancer-Associated Adipocytes Exhibit an Activated Phenotype and Contribute to Breast Cancer Invasion. Cancer Res. 2011, 71, 2455–2465. [Google Scholar] [CrossRef] [Green Version]
- Duong, M.N.; Geneste, A.; Fallone, F.; Li, X.; Dumontet, C.; Muller, C. The fat and the bad: Mature adipocytes, key actors in tumor progression and resistance. Oncotarget 2017, 8, 57622–57641. [Google Scholar] [CrossRef] [Green Version]
- Carter, J.C.; Church, F.C. Mature breast adipocytes promote breast cancer cell motility. Exp. Mol. Pathol. 2012, 92, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Suganami, T.; Ogawa, Y. Adipose tissue macrophages: Their role in adipose tissue remodeling. J. Leukoc. Biol. 2010, 88, 33–39. [Google Scholar] [CrossRef]
- Qiu, S.Q.; Waaijer, S.J.; Zwager, M.C.; de Vries, E.G.; van der Vegt, B.; Schröder, C.P. Tumor-associated macrophages in breast cancer: Innocent bystander or important player? Cancer Treat. Rev. 2018, 70, 178–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Sekiya, I.; Larson, B.L.; Vuoristo, J.T.; Cui, J.-G.; Prockop, D.J. Adipogenic Differentiation of Human Adult Stem Cells From Bone Marrow Stroma (MSCs). J. Bone Miner. Res. 2003, 19, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Bosco, D.B.; Kenworthy, R.; Zorio, D.A.R.; Sang, Q.-X.A. Human Mesenchymal Stem Cells Are Resistant to Paclitaxel by Adopting a Non-Proliferative Fibroblastic State. PLoS ONE 2015, 10, e0128511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sintiprungrat, K.; Singhto, N.; Sinchaikul, S.; Chen, S.-T.; Thongboonkerd, V. Alterations in cellular proteome and secretome upon differentiation from monocyte to macrophage by treatment with phorbol myristate acetate: Insights into biological processes. J. Proteom. 2010, 73, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-G.; Ryoo, I.-G.; Choi, H.-Y.; Choi, B.-H.; Kim, S.-T.; Heo, T.-H.; Lee, J.Y.; Park, P.-H.; Kwak, M.-K. NRF2 Signaling Negatively Regulates Phorbol-12-Myristate-13-Acetate (PMA)-Induced Differentiation of Human Monocytic U937 Cells into Pro-Inflammatory Macrophages. PLoS ONE 2015, 10, e0134235. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Andarawewa, K.L.; Motrescu, E.R.; Chenard, M.-P.; Gansmuller, A.; Stoll, I.; Tomasetto, C.; Rio, M.-C. Stromelysin-3 Is a Potent Negative Regulator of Adipogenesis Participating to Cancer Cell-Adipocyte Interaction/Crosstalk at the Tumor Invasive Front. Cancer Res. 2005, 65, 10862–10871. [Google Scholar] [CrossRef] [Green Version]
- Lijnen, H.R.; Van, H.B.; Frederix, L.; Rio, M.C.; Collen, D. Adipocyte hypertrophy in stromelysin-3 deficient mice with nutritionally induced obesity. Thromb. Haemost. 2002, 87, 530–535. [Google Scholar]
- Maquoi, E.; Demeulemeester, D.; Vörös, G.; Collen, D.; Lijnen, H.R. Enhanced nutritionally induced adipose tissue development in mice with stromelysin-1 gene inactivation. Thromb. Haemost. 2003, 89, 696–704. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Lehuédé, C.; Laurent, V.; Dirat, B.; Dauvillier, S.; Bochet, L.; Le Gonidec, S.; Escourrou, G.; Valet, P.; Muller, C. Adipose tissue and breast epithelial cells: A dangerous dynamic duo in breast cancer. Cancer Lett. 2012, 324, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbaramaiah, K.; Howe, L.R.; Bhardwaj, P.; Du, B.; Gravaghi, C.; Yantiss, R.K.; Zhou, X.K.; Blaho, V.A.; Hla, T.; Yang, P.; et al. Obesity Is Associated with Inflammation and Elevated Aromatase Expression in the Mouse Mammary Gland. Cancer Prev. Res. 2011, 4, 329–346. [Google Scholar] [CrossRef] [Green Version]
- Permana, P.A.; Menge, C.; Reaven, P.D. Macrophage-secreted factors induce adipocyte inflammation and insulin resistance. Biochem. Biophys. Res. Commun. 2006, 341, 507–514. [Google Scholar] [CrossRef]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M. Adipose tissue, obesity and adipokines: Role in cancer promotion. Horm. Mol. Biol. Clin. Investig. 2015, 21, 57–74. [Google Scholar] [CrossRef]
- Hauner, H.; Petruschke, T.; Russ, M.; Röhrig, K.; Eckel, J. Effects of tumour necrosis factor alpha (TNFα) on glucose transport and lipid metabolism of newly-differentiated human fat cells in cell culture. Diabetologia 1995, 38, 764–771. [Google Scholar] [CrossRef] [Green Version]
- Petruschke, T.; Hauner, H. Tumor necrosis factor-alpha prevents the differentiation of human adipocyte precursor cells and causes delipidation of newly developed fat cells. J. Clin. Endocrinol. Metab. 1993, 76, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Hanby, A.; Dublin, E.; Poulsom, R.; Smith, P.; Barnes, D.; Rubens, R.; Anglard, P.; Hart, I. Stromelysin 3: An independent prognostic factor for relapse-free survival in node-positive breast cancer and demonstration of novel breast carcinoma cell expression. Am. J. Pathol. 1998, 152, 721–728. [Google Scholar]





| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| CD 11b | 5′-CTTCCAGGTTCTGGCTCCTTC-3′ | 5′-TCTCTTGGAAGGTCATTGCGTT-3′ |
| MCP-1 | 5′-TCTCAAACTGAAGCTCGCACT-3′ | 5′-GGGAATGAAGGTGGCTGCTA-3′ |
| IL-6 | 5′-CCTGCAGAAAAAGGCAAAGAATC-3′ | 5′-GAGTTGTCATGTCCTGCAGCC-3′ |
| RETN | 5′-TGCAGGATGAAAGCTCTCTGTCTC-3′ | 5′-CCTGGATCCTCTCATTGATGGC-3′ |
| TNF-α | 5′-TGGGATCATTGCCCTGTGAG-3′ | 5′-GGTGTCTGAAGGAGGGGGTA-3′ |
| IL-1β | 5′-CAGGCTGCTCTGGGATTCTC-3′ | 5′-AAGTCATCCTCATTGCCACTGT-3′ |
| AdipoQ | 5′-ATGGCCCCTGCACTACTCTA-3′ | 5′-CAGGGATGAGTTCGGCACTT-3′ |
| LEP | 5′-CCCTGGAGTGCAGTTTCCAA-3′ | 5′-TGCTCAGATGAACCCAACCC-3′ |
| PAI-1 | 5′-TTGCAGGATGGAACTACGGG-3′ | 5′-GTGGCAGGCAGTACAAGAGTGA-3′ |
| MMP-3 | 5′-CCATCTCTTCCTTCAGGCGT-3′ | 5′-ATGCCTCTTGGGTATCCAGC-3′ |
| MMP-11 | 5′-ATGAATTTGGCCACGTGCTG-3′ | 5′-CGAAAGGTGTAGAAGGCGGA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallega, K.A.; Bosco, D.B.; Ren, Y.; Sang, Q.-X.A. Macrophage-Conditioned Media Promotes Adipocyte Cancer Association, Which in Turn Stimulates Breast Cancer Proliferation and Migration. Biomolecules 2022, 12, 1757. https://doi.org/10.3390/biom12121757
Vallega KA, Bosco DB, Ren Y, Sang Q-XA. Macrophage-Conditioned Media Promotes Adipocyte Cancer Association, Which in Turn Stimulates Breast Cancer Proliferation and Migration. Biomolecules. 2022; 12(12):1757. https://doi.org/10.3390/biom12121757
Chicago/Turabian StyleVallega, Karin A., Dale B. Bosco, Yi Ren, and Qing-Xiang Amy Sang. 2022. "Macrophage-Conditioned Media Promotes Adipocyte Cancer Association, Which in Turn Stimulates Breast Cancer Proliferation and Migration" Biomolecules 12, no. 12: 1757. https://doi.org/10.3390/biom12121757