Replacement of the Trabecular Meshwork Cells—A Way Ahead in IOP Control?
Abstract
:1. Introduction
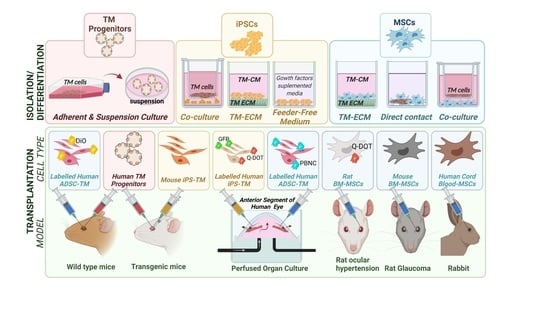
2. Cell Type
2.1. The TM Cell
2.2. The TM Progenitor Cell
- (i)
- Utilising resident stem cells to repopulate the adult population of cells is an attractive option, as it is a process that is prevalent throughout the body, including the eye (e.g., limbus of the cornea epithelium). However, as with other structures in the eye (such as corneal endothelium), this process is thought to not occur (or to happen only in a very limited manner) in the human TM in situ. In 1982, Raviola et al. first identified a new cell type in Schwalbe’s line. This fourth region of the TM (also known as the insert or the transition zone) does not filter aqueous humour, and evidence suggests that stem cells or stem-like progenitor cells reside in this area and that these cells are able to replace TM cells when damage occurs [7,25,26,27]. Cells found in this area have different ultrastructure compared to surrounding TM cells, such as a prominent Golgi apparatus and secretory granules [28]. In addition, Acott et al. reported that surgical insult caused by laser trabeculoplasty initiated cell division by a population of cells in the Schwalbe’s line region to migrate and repopulate the injured sites [29]. Further studies by McGowan et al. found that specific stem cell markers, such as OCT3/4, Wnt-1, PAX6, and SOX2, are expressed in the transition zone between the TM and the corneal endothelium, suggesting that a stem-like cell population may have the potential to repopulate either the TM or the corneal endothelial cells [26]. As a stem-like cell population serves to be on standby to repopulate the TM when damage occurs, the reason why these cells do not repopulate the TM in age and glaucoma when TM cell numbers decrease remains unknown [17,27,30]. It is not unreasonable to hypothesise that changes in genetic, structural, and mechanical properties in the TM occurring as a result of glaucoma may be preventing these progenitor cells from differentiating to replace lost TM cells or actually become lost themselves. Indeed, recently, Sundaresen and colleagues observed that there were reduced numbers of cells with specific stem cell markers ABCG2, neural crest marker p75, and AnkG in older patients compared to younger patients and that the reduced stem/progenitor cell numbers corelated with a reduction in TM tissue cellularity overall [31]. To say that a singular stem cell population exists in one area of the TM may be over simplistic. Not only does it overlook cellular plasticity, but it would also differ from elsewhere in the body. Stem cell populations are largely heterogeneous compared to other cell subpopulations [32]. This can make it difficult to fully understand their physiology and predict their path of differentiation, hindering their use in studies that rely on differentiation into specific cell types due to low efficiency [33]. Heterogeneity of cell types is influenced by tissue physiology and pathology [34] and, thus, within a small area, different sub-populations of stem cells can be present at one time due to differing external microenvironments. In a tissue like the TM, for example, cells are exposed to variations in pressure throughout the day which may not be uniform across the tissue due to the segmental nature of aqueous humour outflow [8,35]. The TM also has distinct regions with different properties such as differing extracellular matrix components, structure, and flexibility [36], which also influences how cells respond to specific growth factors such as transforming growth factor beta (TGFβ) (see also [8,9,37,38,39,40,41]). Therefore, the expression profile of cells in different areas of the TM at any given time are most likely very different and require further understanding of the distinct sub populations of cells within the tissue. This challenge is being addressed with differential harvesting of the sub-populations of SC from TM (outlined in below section about isolation and expansion of TM progenitor cells). In addition, scRNAseq technologies are being utilised to identify heterogeneous sub-populations and observe developmental trajectories of individual stem cells in healthy development and disease [32,42]. The ability to map certain genes to sub-populations of stem cells facilitates tracking the trajectory of which stem cell populations differentiate into terminally differentiated cell types within a tissue [32]. This may be crucial in understanding (and thus making use of potential clinical benefits) as well as identifying disease-associated genes within specific stem cell sub-populations [32].
- (ii)
- Isolation and Expansion of TM Progenitor Cells
- (a)
- Side population cell sorting
- (b)
- Neurosphere assay
- (iii)
- Identification of TM progenitor cells
2.3. Induced Pluripotent Stem Cells Derived TM cells (IPSC-TM Cells)
2.4. Mesenchymal Stem Cell Derived TM Cells (MSC-TM Cells)
3. Transplantation Studies
3.1. Adult TM Cell Transplantation
3.2. TM Progenitor Cell Transplantation
3.3. iPS Cell Transplantation
3.4. MSCs Transplantation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, B.; McNally, S.; Kilpatrick, J.; Jarvis, S.P.; O’Brien, C.J. Aging and ocular tissue stiffness in glaucoma. Surv. Ophthalmol. 2018, 63, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.H.; Fingert, J.H.; Kuehn, M.H.; Alward, W.L. Primary open-angle glaucoma. N. Engl. J. Med. 2009, 360, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K.; Wilson, M.R.; Gordon, M.O. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 701–713. [Google Scholar] [CrossRef]
- Kass, M.A.; Gordon, M.O.; Gao, F.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.K.; Miller, M.J.P.; Parrish, R.K.; Wilson, M.R. Delaying treatment of ocular hypertension: The ocular hypertension treatment study. Arch. Ophthalmol. 2010, 128, 276. [Google Scholar]
- Van Buskirk, E.M. The anatomy of the limbus. Eye 1989, 3, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamm, E.R. The trabecular meshwork outflow pathways: Structural and functional aspects. Exp. Eye Res. 2009, 88, 648–655. [Google Scholar] [CrossRef]
- Yun, H.; Zhou, Y.; Wills, A.; Du, Y. Stem Cells in the Trabecular Meshwork for Regulating Intraocular Pressure. J. Ocul. Pharmacol. Ther. 2016, 32, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Vranka, J.A.; Bradley, J.M.; Yang, Y.-F.; Keller, K.E.; Acott, T.S. Mapping Molecular Differences and Extracellular Matrix Gene Expression in Segmental Outflow Pathways of the Human Ocular Trabecular Meshwork. PLoS ONE 2015, 10, e0122483. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Read, A.T.; Sulchek, T.; Ethier, C.R. Trabecular meshwork stiffness in glaucoma. Exp. Eye Res. 2017, 158, 3–12. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Robinson, M.R.; Dibas, M.; Stamer, W.D. Matrix Metalloproteinases and Glaucoma Treatment. J. Ocul. Pharmacol. Ther. 2020, 36, 208–228. [Google Scholar] [CrossRef]
- Stamer, W.D.; Clark, A.F. The many faces of the trabecular meshwork cell. Exp. Eye Res. 2017, 158, 112–123. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, M.; Zhang, Z.; Xue, H.; Chen, X.; Ji, Y. Physiological function of myocilin and its role in the pathogenesis of glaucoma in the trabecular meshwork (Review). Int. J. Mol. Med. 2018, 43, 671–681. [Google Scholar] [CrossRef]
- Kuchtey, J.; Kallberg, M.E.; Gelatt, K.N.; Rinkoski, T.; Komaromy, A.M.; Kuchtey, R.W. Angiopoietin-like 7 Secretion Is Induced by Glaucoma Stimuli and Its Concentration Is Elevated in Glaucomatous Aqueous Humor. Invest. Ophthalmol. Vis. Sci. 2008, 49, 3438–3448. [Google Scholar] [CrossRef]
- Elliott, M.H.; Ashpole, N.E.; Gu, X.; Herrnberger, L.; McClellan, M.E.; Griffith, G.L.; Reagan, A.M.; Boyce, T.M.; Tanito, M.; Tamm, E.R.; et al. Caveolin-1 modulates intraocular pressure: Implications for caveolae mechanoprotection in glaucoma. Sci. Rep. 2016, 6, 37127. [Google Scholar] [CrossRef] [Green Version]
- Keller, K.E.; Bhattacharya, S.K.; Borrás, T.; Brunner, T.M.; Chansangpetch, S.; Clark, A.F.; Dismuke, W.M.; Du, Y.; Elliott, M.H.; Ethier, C.R.; et al. Consensus recommendations for trabecular meshwork cell isolation, characterization and culture. Exp. Eye Res. 2018, 171, 164–173. [Google Scholar] [CrossRef]
- Liton, P.B.; Challa, P.; Stinnett, S.; Luna, C.; Epstein, D.L.; Gonzalez, P. Cellular senescence in the glaucomatous outflow pathway. Exp. Gerontol. 2005, 40, 745–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, P.; Caballero, M.; Liton, P.B.; Stamer, W.D.; Epstein, D.L. Expression analysis of the matrix GLA protein and VE-cadherin gene promoters in the outflow pathway. Invest. Ophthalmol. Vis. Sci. 2004, 45, 1389–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamer, W.D.; Chan, D.W.; Conley, S.M.; Coons, S.; Ethier, C.R. Aquaporin-1 expression and conventional aqueous outflow in human eyes. Exp. Eye Res. 2008, 87, 349–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, G.; Fury, W.; Yang, H.; Gomez-Caraballo, M.; Bai, Y.; Yang, T.; Adler, C.; Wei, Y.; Ni, M.; Schmitt, H.; et al. Molecular taxonomy of human ocular outflow tissues defined by single-cell transcriptomics. Proc. Natl. Acad. Sci. USA 2020, 117, 12856–12867. [Google Scholar] [CrossRef]
- Shuman, M.A.; Polansky, J.R.; Merkel, C.; Alvarado, J.A. Tissue plasminogen activator in cultured human trabecular meshwork cells. Predominance of enzyme over plasminogen activator inhibitor. Invest. Ophthalmol. Vis. Sci. 1988, 29, 401–405. [Google Scholar] [PubMed]
- Johnson, D.H.; Richardson, T.M.; Epstein, D.L. Trabecular meshwork recovery after phagocytic challenge. Curr. Eye Res. 1989, 8, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, D.A.; Gelatt, K.N.; Gum, G.G. Kinetics of phagocytosis in the normal canine iridocorneal angle. Am. J. Veter. Res. 1984, 45, 2359–2366. [Google Scholar]
- Keller, K.E.; Acott, T.S. The Juxtacanalicular Region of Ocular Trabecular Meshwork: A Tissue with a Unique Extracellular Matrix and Specialized Function. J. Ocul. Biol. Dis. Inform. 2013, 1, 3. [Google Scholar]
- Whikehart, D.R.; Parikh, C.H.; Vaughn, A.V.; Mishler, K.; Edelhauser, H.F. Evidence suggesting the existence of stem cells for the human corneal endothelium. Mol. Vis. 2005, 11, 816–824. [Google Scholar] [PubMed]
- McGowan, S.L.; Edelhauser, H.F.; Pfister, R.R.; Whikehart, D.R. Stem cell markers in the human posterior limbus and corneal endothelium of unwounded and wounded corneas. Mol. Vis. 2007, 13, 1984–2000. [Google Scholar]
- Yu, W.Y.; Sheridan, C.; Grierson, I.; Mason, S.; Kearns, V.; Lo, A.C.Y.; Wong, D. Progenitors for the Corneal Endothelium and Trabecular Meshwork: A Potential Source for Personalized Stem Cell Therapy in Corneal Endothelial Diseases and Glaucoma. J. Biomed. Biotechnol. 2011, 2011, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Raviola, G. Schwalbe line’s cells: A new cell type in the trabecular meshwork of Macaca mulatta. Invest. Ophthalmol. Vis. Sci. 1982, 22, 45–56. [Google Scholar]
- Samples, J.R.; Bradley, J.M.; Bacon, D.R.; Bylsma, S.S.; Van Buskirk, E.M.; Acott, T.S. Trabecular Repopulation by Anterior Trabecular Meshwork Cells After Laser Trabeculoplasty. Am. J. Ophthalmol. 1989, 107, 1–6. [Google Scholar] [CrossRef]
- Keller, K.E.; Aga, M.; Bradley, J.M.; Kelley, M.J.; Acott, T.S. Extracellular matrix turnover and outflow resistance. Exp. Eye Res. 2009, 88, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Sundaresan, Y.; Veerappan, M.; Ramasamy, K.S.; Chidambaranathan, G.P. Identification, quantification and age-related changes of human trabecular meshwork stem cells. Eye Vis. 2019, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liu, L. Applications of single cell RNA sequencing to research of stem cells. World J. Stem Cells 2019, 11, 722–728. [Google Scholar] [CrossRef]
- Han, X.; Chen, H.; Huang, D.; Chen, H.; Fei, L.; Cheng, C.; Huang, H.; Yuan, G.-C.; Guo, G. Mapping human pluripotent stem cell differentiation pathways using high throughput single-cell RNA-sequencing. Genome Biol. 2018, 19, 1–19. [Google Scholar] [CrossRef]
- Wen, L.; Tang, F. Single-cell sequencing in stem cell biology. Genome Biol. 2016, 17, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamer, W.D.; Acott, T.S. Current understanding of conventional outflow dysfunction in glaucoma. Curr. Opin. Ophthalmol. 2012, 23, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Abu-Hassan, D.W.; Acott, T.S.; Kelley, M.J. The Trabecular Meshwork: A Basic Review of Form and Function. J. Ocul. Biol. 2014, 2, 1. [Google Scholar] [CrossRef]
- Vranka, J.A.; Staverosky, J.A.; Reddy, A.P.; Wilmarth, P.A.; David, L.L.; Acott, T.S.; Russell, P.; Raghunathan, V.K. Biome-chanical rigidity and quantitative proteomics analysis of segmental regions of the trabecular meshwork at physiologic and elevated pressures. Invest. Ophthalmol. Vis. Sci. 2018, 59, 246–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy-Ullrich, J.E.; Downs, J.C. The Thrombospondin1-TGF-β Pathway and Glaucoma. J. Ocul. Pharmacol. Ther. 2015, 31, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Braunger, B.M.; Fuchshofer, R.; Tamm, E.R. The aqueous humor outflow pathways in glaucoma: A unifying concept of disease mechanisms and causative treatment. Eur. J. Pharm. Biopharm. 2015, 95, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Villarreal, G., Jr.; Rhee, D.J. Matricellular proteins in the trabecular meshwork: Review and update. J. Ocul. Pharmacol. Ther. 2014, 30, 447–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, S.M.; Sheridan, C.; Kearns, V.R.; Bilir, E.K.; Fan, X.; Grierson, I.; Choudhary, A. Thrombospondin-2 is up-regulated by TGFβ2 and increases fibronectin expression in human trabecular meshwork cells. Exp. Eye Res. 2019, 189, 107820. [Google Scholar] [CrossRef] [PubMed]
- Zerti, D.; Collin, J.; Queen, R.; Cockell, S.J.; Lako, M. Understanding the complexity of retina and pluripotent stem cell derived retinal organoids with single cell RNA sequencing: Current progress, remaining challenges and future prospective. Curr. Eye Res. 2020, 45, 385–396. [Google Scholar] [CrossRef] [Green Version]
- Nadri, S.; Yazdani, S.; Arefian, E.; Gohari, Z.; Eslaminejad, M.B.; Kazemi, B.; Soleimani, M. Mesenchymal stem cells from trabecular meshwork become photoreceptor-like cells on amniotic membrane. Neurosci. Lett. 2013, 541, 43–48. [Google Scholar] [CrossRef]
- Du, Y.; Roh, D.S.; Mann, M.M.; Funderburgh, M.L.; Funderburgh, J.L.; Schuman, J.S. Multipotent Stem Cells from Trabecular Meshwork Become Phagocytic TM Cells. Invest. Ophthalmol. Vis. Sci. 2012, 53, 1566–1575. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Yun, H.; Yang, E.; Schuman, J. Stem Cells from Trabecular Meshwork Home to TM Tissue In Vivo. Invest. Ophthalmol. Vis. Sci. 2013, 54, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Wang, Y.; Zhou, Y.; Kumar, A.; Wang, K.; Sun, M.; Stolz, D.B.; Xia, X.; Ethier, C.R.; Du, Y. Human stem cells home to and repair laser-damaged trabecular meshwork in a mouse model. Commun. Biol. 2018, 1, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Cheng, T.; Song, W.; Cheuk, B.; Yang, E.; Yang, L.; Xie, Y.; Du, Y. Two-step induction of trabecular meshwork cells from induced pluripotent stem cells for glaucoma. Biochem. Biophys. Res. Commun. 2020, 529, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Xu, Y.; Wang, Y.; Kumar, A.; Peters, D.M.; Du, Y. α5β1 Integrin Promotes Anchoring and Integration of Transplanted Stem Cells to the Trabecular Meshwork in the Eye for Regeneration. Stem Cells Dev. 2020, 29, 290–300. [Google Scholar] [CrossRef]
- Gonzalez, P.; Epstein, D.; Luna, C.; Liton, P. Characterization of free-floating spheres from human trabecular meshwork (HTM) cell culture in vitro. Exp. Eye Res. 2006, 82, 959–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.Y.; Grierson, I.; Sheridan, C.; Lo, A.C.-Y.; Wong, D.S.-H. Bovine Posterior Limbus: An Evaluation of an Alternative Source for Corneal Endothelial and Trabecular Meshwork Stem/Progenitor Cells. Stem Cells Dev. 2015, 24, 624–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Cai, S.; Tseng, S.C.; Zhu, Y.-T. Isolation and expansion of multipotent progenitors from human trabecular mesh-work. Sci. Rep. 2018, 8, 1–9. [Google Scholar]
- Tay, C.Y.; Sathiyanathan, P.; Chu, S.W.; Stanton, L.W.; Wong, T.T. Identification and Characterization of Mesenchymal Stem Cells Derived from the Trabecular Meshwork of the Human Eye. Stem Cells Dev. 2012, 21, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Sathiyanathan, P.; Tay, C.Y.; Stanton, L.W. Transcriptome analysis for the identification of cellular markers related to trabecular meshwork differentiation. BMC Genom. 2017, 18, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yam, G.H.-F.; Seah, X.; Yusoff, N.Z.B.M.; Setiawan, M.; Wahlig, S.; Htoon, H.M.; Peh, G.S.; Kocaba, V.; Mehta, J.S. Character-ization of human transition zone reveals a putative progenitor-enriched niche of corneal endothelium. Cells 2019, 8, 1244. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ahmad, A.M.; Ng, H.; Shi, J.; McGhee, C.N.J.; Patel, D.V. Successful culture of human transition zone cells. Clin. Exp. Ophthalmol. 2020, 48, 689–700. [Google Scholar] [CrossRef]
- Golebiewska, A.; Brons, N.H.; Bjerkvig, R.; Niclou, S. Critical Appraisal of the Side Population Assay in Stem Cell and Cancer Stem Cell Research. Cell Stem Cell 2011, 8, 136–147. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Morshead, C.M. Distinct Populations of Forebrain Neural Stem and Progenitor Cells Can Be Isolated Using Side-Population Analysis. J. Neurosci. 2003, 23, 10703–10709. [Google Scholar] [CrossRef]
- Branch, M.J.; Yu, W.-Y.; Sheridan, C.; Hopkinson, A. Isolation of Adult Stem Cell Populations from the Human Cornea. Adv. Struct. Saf. Stud. 2014, 1235, 165–177. [Google Scholar] [CrossRef]
- Reynolds, B.; Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 1992, 255, 1707–1710. [Google Scholar] [CrossRef] [Green Version]
- Pastrana, E.; Silva-Vargas, V.; Doetsch, F. Eyes Wide Open: A Critical Review of Sphere-Formation as an Assay for Stem Cells. Cell Stem Cell 2011, 8, 486–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doetsch, F.; Petreanu, L.; Caillé, I.; García-Verdugo, J.M.; Alvarez-Buylla, A. EGF Converts Transit-Amplifying Neurogenic Precursors in the Adult Brain into Multipotent Stem Cells. Neuron 2002, 36, 1021–1034. [Google Scholar] [CrossRef] [Green Version]
- Stamer, W.D.; Seftor, R.E.B.; Williams, S.K.; Samaha, H.A.M.; Snyder, R.W. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr. Eye Res. 1995, 14, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Lendahl, U.; Zimmerman, L.B.; McKay, R.D.G. CNS stem cells express a new class of intermediate filament protein. Cell 1990, 60, 585–595. [Google Scholar] [CrossRef]
- Pitman, M.; Emery, B.; Binder, M.; Wang, S.; Butzkueven, H.; Kilpatrick, T. LIF receptor signaling modulates neural stem cell renewal. Mol. Cell. Neurosci. 2004, 27, 255–266. [Google Scholar] [CrossRef]
- Xie, H.-T.; Chen, S.-Y.; Li, G.-G.; Tseng, S.C.G. Isolation and Expansion of Human Limbal Stromal Niche Cells. Invest. Ophthalmol. Vis. Sci. 2012, 53, 279–286. [Google Scholar] [CrossRef]
- Qutub, A.; Popel, A.S. Elongation, proliferation & migration differentiate endothelial cell phenotypes and determine capillary sprouting. BMC Syst. Biol. 2009, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef] [PubMed]
- van Zyl, T.; Yan, W.; McAdams, A.; Peng, Y.-R.; Shekhar, K.; Regev, A.; Juric, D.; Sanes, J.R. Cell atlas of aqueous humor outflow pathways in eyes of humans and four model species provides insight into glaucoma pathogenesis. Proc. Natl. Acad. Sci. USA 2020, 117, 10339–10349. [Google Scholar] [CrossRef]
- Ding, Q.J.; Zhu, W.; Cook, A.C.; Anfinson, K.R.; Tucker, B.A.; Kuehn, M.H. Induction of trabecular meshwork cells from in-duced pluripotent stem cells. Invest. Ophthalmol. Vis. Sci. 2014, 55, 7065–7072. [Google Scholar] [CrossRef] [Green Version]
- Abu-Hassan, D.W.; Li, X.; Ryan, E.I.; Acott, T.S.; Kelley, M.J. Induced Pluripotent Stem Cells Restore Function in a Human Cell Loss Model of Open-Angle Glaucoma. Stem Cells 2015, 33, 751–761. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Miao, Y.; Sui, S.; Wang, Y.; Wu, S.; Cao, Q.; Duan, H.; Qi, X.; Zhou, Q.; Pan, X.; et al. Xeno- and Feeder-Free Differentiation of Human iPSCs to Trabecular Meshwork-Like Cells by Recombinant Cytokines. Transl. Vis. Sci. Technol. 2021, 10, 27. [Google Scholar] [CrossRef]
- Tian, Y.I.; Zhang, X.; Torrejon, K.; Danias, J.; Du, Y.; Xie, Y. A Biomimetic, Stem Cell-Derived In Vitro Ocular Outflow Model. Adv. Biosyst. 2020, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Godwin, C.R.; Cheng, L.; Scheetz, T.E.; Kuehn, M.H. Transplantation of iPSC-TM stimulates division of trabecular meshwork cells in human eyes. Sci. Rep. 2020, 10, 2905–2911. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Jain, A.; Gramlich, O.W.; Tucker, B.A.; Sheffield, V.C.; Kuehn, M.H. Restoration of aqueous humor outflow following transplantation of iPSC-derived trabecular meshwork cells in a transgenic mouse model of glaucoma. Invest. Ophthalmol. Vis. Sci. 2017, 58, 2054–2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Gramlich, O.W.; Laboissonniere, L.; Jain, A.; Sheffield, V.C.; Trimarchi, J.M.; Tucker, B.A.; Kuehn, M.H. Transplan-tation of iPSC-derived TM cells rescues glaucoma phenotypes in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, E3492–E3500. [Google Scholar] [CrossRef] [Green Version]
- Harrell, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, A.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes in the Treatment of Eye Diseases. Adv. Exp. Med. Biol. 2018, 1089, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Chen, G.; Li, Y.; Xie, X.; Zhou, Y.; Du, Z. Aggressive invasion is observed in CD133−/A2B5+ glioma-initiating cells. Oncol. Lett. 2015, 10, 3399–3406. [Google Scholar] [CrossRef] [Green Version]
- Morgan, J.; Wood, J.A.; Walker, N.J.; Raghunathan, V.K.; Borjesson, D.L.; Murphy, C.J.; Russell, P. Human Trabecular Meshwork Cells Exhibit Several Characteristics of, but Are Distinct from, Adipose-Derived Mesenchymal Stem Cells. J. Ocul. Pharmacol. Ther. 2014, 30, 254–266. [Google Scholar] [CrossRef]
- Snider, E.J.; Kubelick, K.P.; Tweed, K.; Kim, R.K.; Li, Y.; Gao, K.; Read, A.T.; Emelianov, S.; Ethier, C.R. Improving Stem Cell Delivery to the Trabecular Meshwork Using Magnetic Nanoparticles. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Manuguerra-Gagné, R.; Boulos, P.R.; Ammar, A.; Leblond, F.A.; Krosl, G.; Pichette, V.; Lesk, M.R.; Roy, D.-C. Transplantation of Mesenchymal Stem Cells Promotes Tissue Regeneration in a Glaucoma Model Through Laser-Induced Paracrine Factor Secretion and Progenitor Cell Recruitment. Stem Cells 2013, 31, 1136–1148. [Google Scholar] [CrossRef]
- Du, Y.; Roh, D.S.; Funderburgh, M.L.; Mann, M.M.; Marra, K.; Rubin, J.P.; Li, X.; Funderburgh, J.L. Adipose-derived stem cells differentiate to keratocytes in vitro. Mol. Vis. 2010, 16, 2680–2689. [Google Scholar]
- Zhou, Y.; Xia, X.; Yang, E.; Wang, Y.; Marra, K.G.; Ethier, C.R.; Schuman, J.S.; Du, Y. Adipose-derived stem cells integrate into trabecular meshwork with glaucoma treatment potential. FASEB J. 2020, 34, 7160–7177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, S.; Kumar, A.; Tian, S.E.; Taher, E.; Yang, E.; Kinchington, P.R.; Xia, X.; Du, Y. Stem cell transplantation rescued a primary open-angle glaucoma mouse model. eLife 2021, 10, 63677. [Google Scholar] [CrossRef] [PubMed]
- Roubeix, C.; Godefroy, D.; Mias, C.; Sapienza, A.; Riancho, L.; Degardin, J.; Fradot, V.; Ivkovic, I.; Picaud, S.; Sennlaub, F.; et al. Intraocular pressure reduction and neuroprotection conferred by bone marrow-derived mesenchymal stem cells in an animal model of glaucoma. Stem Cell Res. Ther. 2015, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sihota, R.; Sen, S.; Mohanty, S.; Ahmad, M.; Ravi, A.; Gupta, V.; Bhatla, N. Effect of intracameral human cord blood-derived stem cells on lasered rabbit trabecular meshwork. Int. Ophthalmol. 2019, 39, 2757–2766. [Google Scholar] [CrossRef]
- Sugita, S.; Mandai, M.; Hirami, Y.; Takagi, S.; Maeda, T.; Fujihara, M.; Matsuzaki, M.; Yamamoto, M.; Iseki, K.; Hayashi, N.; et al. HLA-Matched Allogeneic iPS Cells-Derived RPE Transplantation for Macular Degeneration. J. Clin. Med. 2020, 9, 2217. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Zode, G.; Kasetti, R.B.; Ran, F.A.; Yan, W.; Sharma, T.P.; Bugge, K.; Searby, C.C.; Fingert, J.H.; Zhang, F.; et al. CRISPR-Cas9–based treatment of myocilin-associated glaucoma. Proc. Natl. Acad. Sci. USA 2017, 114, 11199–11204. [Google Scholar] [CrossRef] [Green Version]
- Petit, I.; Szyper-Kravitz, M.; Nagler, A.; Lahav, M.; Peled, A.; Habler, L.; Ponomaryov, T.; Taichman, R.S.; Arenzana-Seisdedos, F.; Fujii, N.; et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR. Nat. Immunol. 2002, 3, 687–694. [Google Scholar] [CrossRef]
| Ref | Method of TM Progenitor Cell Isolation | TM Progenitor Cell Expansion Medium | Similar Methods |
|---|---|---|---|
| [42] | Cells were isolated from the TM tissue and cultured in specific culture medium on adherent culture conditions for sorting the TM progenitor cells | Low glucose DMEM supplemented with 20% serum and 200 ng/mL basic-FGF | N/A |
| [43] | Cells were isolated from the TM tissue. TM progenitor cells were sorted from TM cells by SP assay and cultured in stem cell medium on adherent culture conditions | Stem cell growth medium (SCGM) containing multipurpose reduced-serum media (Opti-MEM) supplemented with 5% fetal bovine serum (FBS), 10 ng/mL epidermal growth factor (EGF), 100 μg/mL bovine pituitary extract, 20 μg/mL ascorbic acid, 200 μg/mL calcium chloride, 0.08% chondroitin sulfate, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 50 μg/mL gentamicin | [44,45,46,47] |
| [48] | Cells isolated from TM tissue were cultured in stem cell culture medium in suspension. TM progenitor cells were isolated by sphere formation assay | StemSpan™ Serum-Free Expansion Medium (SFEM) (StemCell Technologies, Seattle, WA, USA) | N/A |
| [49] | Cells were isolated from the TM tissue and cultured in neural stem cell culture medium in suspension. TM progenitor cells were isolated by sphere formation assay | DMEM (low glucose), containing 20 ng/mL EGF (Peprotech, UK), 20 ng/mL b-FGF (Peprotech, UK), 5 ug/mL Heparin Sodium (Sigma-Aldrich, UK) and 1X B27 supplement (Thermo Fisher Scientific, UK) at 37 °C, 5% CO2 | [26] |
| [50] | Cells were isolated from the TM tissue and cultured in 3D Matrigel with stem cell culture medium. TM progenitor cells were isolated by sphere formation assay | 3D Matrigel made by adding 50% diluted Matrigel in MESCM + 5% FBS | N/A |
| [51] | Cells were isolated from the TM tissue and cultured in specific culture medium on adherent culture conditions for sorting the TM progenitor cells | DMEM (low glucose), containing 10% FBS, 4 mM L-GlutaMAX™, 1 mM sodium pyruvate, 1% nonessential amino acids, and 1% penicillin–streptomycin, on uncoated tissue culture plastic. | [52] |
| [53] | Cells isolated from the transition zone between TM and cornea endothelium (the tissue without pigmented TM and translucent peripheral endothelium) were cultured in the specific culture medium in 2D Matrigel | OptiMEM1 with recombinant human epidermal growth factor (h-EGF, 10 ng/mL), recombinant human basic fibroblast growth factor (h-bFGF, 20 ng/mL), bovine pituitary extract (100 μg/mL), L-ascorbate (20 μg/mL), chondroitin sulfate (0.08%), calcium chloride (0.9 mM), and knockout serum replacement (5%). | [54] |
| Ref | TM Progenitor Cell Culture Conditions | Detection Method | TM Progenitor Cell Markers |
|---|---|---|---|
| [42] | Adherent culture conditions | Flow cytometric analysis | CD105, CD90, CD44, and CD166 |
| [43] | Adherent culture conditions | PCR and/or immunofluorescence | ABCG2, NOTCH-1, MUC1, and ANKG |
| [47] | Adherent culture conditions | PCR, western blotting, immunofluorescence, and/or flow cytometric analysis | NES, OCT3/4, α5 integrin, and α5β1 integrin |
| [48] | Suspension conditions (sphere formation) | Microarray and PCR | NES, LIF, BDNF, IL6, and CSF3 |
| [50] | Suspension conditions (sphere formation in 3D Matrigel) | PCR and/or immunofluorescence | OCT4, SOX2, KLF4, Vim, AQP1, CHI3L1, MGP, and AnkG |
| [51] | Adherent culture conditions | Flow cytometric analysis and immunocytochemistry | CD73, CD90, and CD105 |
| [54] | Adherent culture conditions | RCR and/or immunofluorescence | ZO-1, Na+/K+ ATPase, PITX2, and SOX10 |
| Ref | Detection Methods | Markers Expressed in the TM Region | Markers Expressed in the TZ Region | Markers Expressed in the SC and JCT Region | Markers Expressed in the PE Region |
|---|---|---|---|---|---|
| [25] | IHC (fluorescence, cryosections) | Unwounded corneas: nestin and telomerase. Post-wounded corneas: SOX2, OCT3/4, Wnt-1, and PAX6 | Unwounded corneas: nestin, alkaline phosphatase, and telomerase. Post-wounded corneas: SOX2, Wnt-1, PAX6, and OCT3/4 | Unwounded corneas: telomerase. Post-wounded corneas: PAX6 and Wnt-1 | Unwounded corneas: nestin and telomerase. Post-wounded corneas: SOX2, OCT3/4, and Wnt-1 |
| [30] | IHC (fluorescence, Paraffin sections) | N/A | P75 and ABCG2 | N/A | N/A |
| [53] | IHC (fluorescence, cryosections) | Vimentin and CD44 | Nestin, Vimentin, Lgr5, SOX2, CD34, HNK1, Prdx6, Pitx2, Lgr5, TERT, P75, and CD44 | N/A | Lgr5, Prdx6, nestin, CD34, and TERT |
| [67] | RNAscope multiplex fluorescent assay | PDPN, DES | PDPN | PDPN, CHI3L1, PECAM, POSTN, TFF3, DES, Pecam1, and POSTN | AOP1 |
| Ref | Model | Differentiation Method | Detection Method | TM Markers | |
|---|---|---|---|---|---|
| [46] | Human | Two step induction (first step NC, then TM-ECM) (10–14 days) | RT-PCR, immunohistochemistry (IHC) | MYOC, CHI3L1, NGFR, HNK1, ANGPTL7 | |
| [68] | Mouse and Human | Co-culturing with TC inserts (21 days) | IHC, phagocytosis, proteomics | COL4A5, MGP, MYOC, TIMP3, MMP3 | |
| [69] | Human and Porcine | Generating EBs and TM-ECM (30 days) | POC, IOP measurement, IHC, phagocytosis, qPCR, WB | AQP1, CHI3L1, WNT1, α3 integrin | |
| [70] | Human | Feeder free differentiation (7 days stage 1 and 14 days stage 2) | RT-PCR, immunocytochemistry (ICC), mRNA sequencing | LAMA4, TIMP3, AQP1, COL4, MYOC | |
| [71] | Human and Porcine | Two step induction (first step NC, then TM-ECM) (10–14 days) | 3D culture, SEM, ICC, western blot (WB), IOP measurement | MYOC, αSMA, Fibronectin, COL4 | |
| [72] | Human | Co-culturing with TC inserts (30 days) | IOP measurement, qPCR, IHC, mRNA sequencing | Vimentin, AQP1, MGP, COL4, MYOC, COL1, TIMP3 | |
| [73] | Mouse | Conditioned TM media (14 days) | TEM, WB, IHC, IOP measurement | MYOC, Calnexin | |
| [74] | Mouse | Conditioned TM media (14 days) | POC, IOP measurement, IHC | LAMA4, TIMP3, MYOC, COL4 | |
| Ref | Model | Cell Type | Output |
|---|---|---|---|
| TM Progenitor Cells | |||
| [44] | (C57BL/6) Wild-type mouse | DiO-Labelled trabecular meshwork stem cells (TMSCs) | TM Markers: CHI3L1 and MUC1 positive (after 1 week). Found: primarily localised to TM and few cells in the iris (up to 4 months). Control cells: fibroblast (CHI3L1 or MUC1) found mainly corneal endothelium and lens epithelium. Additional effects: TMSCs or fibroblasts had no effect on corneal transparency, corneal endothelial morphology, or IOP. Only fibroblast-injected eyes displayed an inflammatory response, with increased CD45 in the TM. |
| [45] | (C57BL/6) Mice with laser-photocoagulation of the TM | DiO-labelled human TMSCs | TM Markers: AQP1 and CHI3L1 detected by IF staining. Found: Mostly at laser treated sites in the TM after 2 weeks and to a lesser extent throughout the TM. Control cells: Fibroblast cells—detected in the iris and to a lesser extent throughout the TM. Additional effects: Structure of laser-damaged TM was restored after 4 weeks only in TMSC-injected mice. TMSC-injected mice also had reduced CD45 and SPARC expression compared to damaged, non-injected controls. |
| [82] | Tg-MYOCY437H POAG mice | DiO-labelled human TMSCs | TM markers: AQP1 and CHI3L1 positive (after 2 months). Found: The DiO-labelled TMSCs were detected at the TM region (up to 2 months). Control: Age-matched wild-type (WT) mice. Additional effect: The IOP of the Tg-MYOCY437H mice was decreased after one month of TMSC transplantation; TMSCs remodelled the extracellular matrix in the Tg-MYOCY437H mice. |
| Mesenchymal Stem Cells from Other Tissues | |||
| [78] | Organ cultured porcine eyes | Human adipose derived MSCs labelled with magnetic Prussian blue nanocubes (PBNC-MSCs) | TM Markers: Not investigated. Found: PBNC-MSCs were detected at significantly higher levels in the TM than unlabelled MSCs after 15 min of exposure to magnets to steer the direction of cells. Control cells: Unlabelled MSCs. Additional effect: Labelled cells evenly distributed around the entire TM circumference after magnet exposure. PBNCs had no adverse effect on MSC viability or multipotency (when differentiating into adipogenic and osteogenic phenotype) in vitro, however, this was not measured in vivo. |
| [79] | Rat glaucoma model (laser induced IOP elevation) | Mouse bone marrow MSCs | TM Markers: MSCs were not found to differentiate—IF staining was negative for AQP1, FN, LM, and PAX6. Found: At the laser damaged site between 24 and 48 h. Control: Laser damaged eyes without MSC injection. Additional effects: BM-MSCs injection caused more efficient reduction of IOP. Eyes injected with MSCs did not present with scarring after 1 month. Conditioned medium from MSC cell culture caused reduced IOP suggesting change due to paracrine secretions of MSC cells. MSCs and conditioned medium also induced proliferation of progenitor cells located at ciliary body. |
| [81] | (C57BL/6) Wild-type mouse | Human DiO-labelled adipose derived stem cells (ADSCs) and ADSC-derived-TM cells (ADSC-TM) | TM markers: CHI3L1 and AQP1. Found: ADSC and ADSC-TM integrated throughout all layers of TM tissue (determined by DiO labelling and IF staining) 30 days after injection to the anterior chamber. Control: Primary ADSCs and fibroblasts Additional effect: IOP and outflow facility were maintained in mice injected with ADSC and ADSC-TM but not in fibroblast control. |
| [83] | Rat ocular hypertension model | Rat Q-Dot labelled bone marrow MSCs | TM markers: COL3, COL4, α-SMA. Found: 24 days after the injection, MSCs were found located near the iridocorneal angle, on the corneal endothelium, and in the TM. Control: Hypertensive eyes injected with media, hypertensive eyes injected MSCs suspension, hypertensive eyes injected with differentiated MSCs suspension, and normotensive eyes injected with MSC suspension. Additional effect: Only the MSCs group decreased the IOP significantly compared with the other groups for 13 days. |
| [84] | Rabbit with laser diode treatment | Human cord blood-derived stem cells (HUCB) | TM Markers: Not investigated. Found: HUCB cells lined trabecular beams confirmed by presence of markers CD34/CD44, and PKH26 labelled cells. Control: Laser-damaged eyes without HUCB injection. Additional effects: HUBC-injected eyes had preserved TM structure and endothelial cellularity compared to damaged, non-injected controls. Neither group showed significantly different IOP to baseline after laser treatment. |
| iPSCs | |||
| [69] | Organ cultured human eyes | Human QDot labelled TM-like iPSCs | TM Markers: AQP1, Chi3L1, WNT, α3 Integrin. Found: TM-like iPSCs integrated into the TM. Control: QDot labelled TM cells, dermal fibroblasts, embryoid bodies, and HUVEC cells. Additional effect: IOP homeostatic response to 2x pressure challenge was restored by transplanting TM cells/TM-like iPSCs. |
| [72] | Adult human donor eyes in perfused organ culture | Human GFP labelled iPSC-TM | TM Markers: MGP, MYOC, Vimentin, AQP1, COL4, and COL1. Found: GFP labelled iPSC-TM cells were not detectable in recipient eyes. Control: The second eye from the same donor was maintained as a non-injected control. Additional effect: Transplantation of iPSC-TM stimulates proliferation of the recipients’ endogenous TM cells in perfusion cultured human eyes from aged donors. In total, 71.4% of iPSC-TM recipients’ eye displayed normal outflow facility for two weeks after transplantation. |
| [73] | Young transgenic mice expressing form of human myocilin (Tg-MYOCY437H) | Mouse iPSC-TM | TM Markers: MYOC and calnexin. Found: IOP was lower and outflow facility was improved compared to untreated controls (12 weeks). Control: Wild type mice and PBS injected Tg-MYOCY437H mice were used as the control group. Additional effect: The number of endogenous TM cells increased. |
| [74] | Young transgenic mice expressing a pathogenic form of human myocilin (Tg-MYOCY437H) | Mouse iPSC-TM | TM Markers: MYOC, TIMP3, LAMA4. Found: Injected iPSC-TM cells integrated into the TM (12 weeks). Injected cells were detected also in the endothelial cell layer of the Schlemm’s canal, the corneal epithelium stroma, and the area surrounding the injection site (9 weeks). Control: Fibroblasts injected transgenic mice, PBS injected transgenic mice, and wild type mice were used as control groups. Additional effect: IOP was significantly reduced in iPSC-TM–treated mice. The cellular density of the TM in transgenic iPSC-TM recipient mice was significantly higher than PBS injected transgenic controls and similar to WT animals. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Bilir, E.K.; Kingston, O.A.; Oldershaw, R.A.; Kearns, V.R.; Willoughby, C.E.; Sheridan, C.M. Replacement of the Trabecular Meshwork Cells—A Way Ahead in IOP Control? Biomolecules 2021, 11, 1371. https://doi.org/10.3390/biom11091371
Fan X, Bilir EK, Kingston OA, Oldershaw RA, Kearns VR, Willoughby CE, Sheridan CM. Replacement of the Trabecular Meshwork Cells—A Way Ahead in IOP Control? Biomolecules. 2021; 11(9):1371. https://doi.org/10.3390/biom11091371
Chicago/Turabian StyleFan, Xiaochen, Emine K. Bilir, Olivia A. Kingston, Rachel A. Oldershaw, Victoria R. Kearns, Colin E. Willoughby, and Carl M. Sheridan. 2021. "Replacement of the Trabecular Meshwork Cells—A Way Ahead in IOP Control?" Biomolecules 11, no. 9: 1371. https://doi.org/10.3390/biom11091371