Influence of Culture Substrates on Morphology and Function of Pulmonary Alveolar Cells In Vitro
Abstract
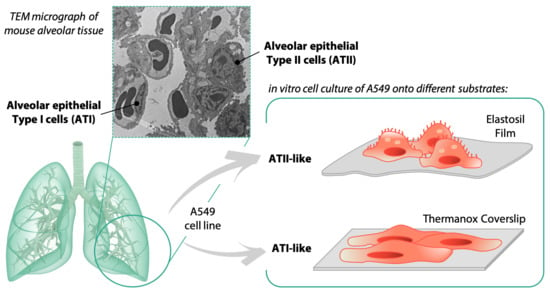
:1. Introduction
2. Materials and Methods
2.1. Characterization of Substrates
2.2. Cells Culture and Experimental Design
2.3. Scanning Electron Microscopy Analysis
2.4. Immunofluorescence
2.5. Statistical Analysis
3. Results
3.1. Characterization of Substrates
3.2. Effect of Substrates on A549 Cells Morphology and Metabolic Activity
3.3. Scanning Electron Microscopy Analysis
3.4. Immunofluorescence Analysis for F-Actin, CASP-3 and Focal Adhesion
3.5. Immunofluorescence Analysis for YAP-1
3.6. Immunofluorescence Analysis for Surfactant Protein C
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crystal, R.G.; Randell, S.H.; Engelhardt, J.F.; Voynow, J.; Sunday, M.E. Airway Epithelial Cells: Current Concepts and Challenges. Proc. Am. Thorac. Soc. 2008, 5, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Crandall, E.D.; Matthay, M.A. Alveolar Epithelial Transport. Basic Science to Clinical Medicine. Am. J. Respir. Crit. Care Med. 2001, 163, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Chuquimia, O.D.; Petursdottir, D.H.; Periolo, N.; Fernández, C. Alveolar Epithelial Cells Are Critical in Protection of the Respiratory Tract by Secretion of Factors Able to Modulate the Activity of Pulmonary Macrophages and Directly Control Bacterial Growth. Infect. Immun. 2013, 81, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Fehrenbach, H. Alveolar Epithelial Type II Cell: Defender of the Alveolus Revisited. Respir. Res. 2001, 2, 33–46. [Google Scholar] [CrossRef]
- Aspal, M.; Zemans, R.L. Mechanisms of ATII-to-ATI Cell Differentiation during Lung Regeneration. Int. J. Mol. Sci. 2020, 21, 3188. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.; Hollins, A.J.; Al-Eid, A.; Newman, G.R.; von Ruhland, C.; Gumbleton, M. Caveolin-1 Expression and Caveolae Biogenesis during Cell Transdifferentiation in Lung Alveolar Epithelial Primary Cultures. Biochem. Biophys. Res. Commun. 1999, 262, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Kawkitinarong, K.; Linz-McGillem, L.; Birukov, K.G.; Garcia, J.G.N. Differential Regulation of Human Lung Epithelial and Endothelial Barrier Function by Thrombin. Am. J. Respir. Cell Mol. Biol. 2004, 31, 517–527. [Google Scholar] [CrossRef]
- Stroetz, R.W.; Vlahakis, N.E.; Walters, B.J.; Schroeder, M.A.; Hubmayr, R.D. Validation of a New Live Cell Strain System: Characterization of Plasma Membrane Stress Failure. J. Appl. Physiol. 2001, 90, 2361–2370. [Google Scholar] [CrossRef]
- Lieber, M.; Smith, B.; Szakal, A.; Nelson-Rees, W.; Todaro, G. A Continuous Tumor-Cell Line from a Human Lung Carcinoma with Properties of Type II Alveolar Epithelial Cells. Int. J. Cancer 1976, 17, 62–70. [Google Scholar] [CrossRef]
- Foster, K.A.; Oster, C.G.; Mayer, M.M.; Avery, M.L.; Audus, K.L. Characterization of the A549 Cell Line as a Type II Pulmonary Epithelial Cell Model for Drug Metabolism. Exp. Cell Res. 1998, 243, 359–366. [Google Scholar] [CrossRef]
- Borok, Z.; Danto, S.I.; Lubman, R.L.; Cao, Y.; Williams, M.C.; Crandall, E.D. Modulation of T1alpha Expression with Alveolar Epithelial Cell Phenotype in Vitro. Am. J. Physiol. 1998, 275, L155–L164. [Google Scholar] [CrossRef] [PubMed]
- Danto, S.I.; Shannon, J.M.; Borok, Z.; Zabski, S.M.; Crandall, E.D. Reversible Transdifferentiation of Alveolar Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 1995, 12, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Figliuzzi, M.; Tironi, M.; Longaretti, L.; Mancini, A.; Teoldi, F.; Sangalli, F.; Remuzzi, A. Copper-Dependent Biological Effects of Particulate Matter Produced by Brake Systems on Lung Alveolar Cells. Arch. Toxicol. 2020, 94, 2965–2979. [Google Scholar] [CrossRef]
- Billet, S.; Landkocz, Y.; Martin, P.J.; Verdin, A.; Ledoux, F.; Lepers, C.; André, V.; Cazier, F.; Sichel, F.; Shirali, P.; et al. Chemical Characterization of Fine and Ultrafine PM, Direct and Indirect Genotoxicity of PM and Their Organic Extracts on Pulmonary Cells. J. Environ. Sci. 2018, 71, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cui, Z. Three-Dimensional Perfused Cell Culture. Biotechnol. Adv. 2014, 32, 243–254. [Google Scholar] [CrossRef]
- Guenat, O.T.; Berthiaume, F. Incorporating Mechanical Strain in Organs-on-a-Chip: Lung and Skin. Biomicrofluidics 2018, 12, 042207. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Tanswell, A.K.; Post, M. Mechanical Force-Induced Signal Transduction in Lung Cells. Am. J. Physiol. 1999, 277, L667–L683. [Google Scholar] [CrossRef]
- Waters, C.M.; Roan, E.; Navajas, D. Mechanobiology in Lung Epithelial Cells: Measurements, Perturbations, and Responses. Compr. Physiol. 2012, 2, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Jansen, K.A.; Donato, D.M.; Balcioglu, H.E.; Schmidt, T.; Danen, E.H.J.; Koenderink, G.H. A Guide to Mechanobiology: Where Biology and Physics Meet. Biochem. Biophys. Acta 2015, 1853, 3043–3052. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Han, D.; Zhao, Y.-P. Kinetic Behaviour of the Cells Touching Substrate: The Interfacial Stiffness Guides Cell Spreading. Sci. Rep. 2015, 4, 3910. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Mih, J.D.; Shea, B.S.; Kho, A.T.; Sharif, A.S.; Tager, A.M.; Tschumperlin, D.J. Feedback Amplification of Fibrosis through Matrix Stiffening and COX-2 Suppression. J. Cell Biol. 2010, 190, 693–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mambetsariev, I.; Tian, Y.; Wu, T.; Lavoie, T.; Solway, J.; Birukov, K.G.; Birukova, A.A. Stiffness-Activated GEF-H1 Expression Exacerbates LPS-Induced Lung Inflammation. PLoS ONE 2014, 9, e92670. [Google Scholar] [CrossRef] [PubMed]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional Homeostasis and the Malignant Phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Shukla, V.C.; Higuita-Castro, N.; Nana-Sinkam, P.; Ghadiali, S.N. Substrate Stiffness Modulates Lung Cancer Cell Migration but Not Epithelial to Mesenchymal Transition: Substrate Stiffness Modulates Lung Cancer Cell Migration. J. Biomed. Mater. Res. 2016, 104, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Xue, C.; Li, Q.; Liu, M.; Ma, W.; Zhou, T.; Lin, Y. Substrate Stiffness Regulated Migration and Angiogenesis Potential of A549 Cells and HUVECs. J. Cell Physiol. 2018, 233, 3407–3417. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.H.; Dysart, M.M.; Brown, A.C.; Douglas, A.M.; Fiore, V.F.; Russell, A.G. HEI Health Review Committee. Synergistic Effects of Particulate Matter and Substrate Stiffness on Epithelial-to-Mesenchymal Transition. Res. Rep. Health Eff Inst. 2014, 182, 3–41. [Google Scholar]
- Mohri, Z.; Del Rio Hernandez, A.; Krams, R. The Emerging Role of YAP/TAZ in Mechanotransduction. J. Thorac. Dis. 2017, 9, E507–E509. [Google Scholar] [CrossRef] [Green Version]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The Biology of YAP/TAZ: Hippo Signaling and Beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef]
- LaCanna, R.; Liccardo, D.; Zhang, P.; Tragesser, L.; Wang, Y.; Cao, T.; Chapman, H.A.; Morrisey, E.E.; Shen, H.; Koch, W.J.; et al. Yap/Taz Regulate Alveolar Regeneration and Resolution of Lung Inflammation. J. Clin. Investig. 2019, 129, 2107–2122. [Google Scholar] [CrossRef] [Green Version]
- Elbediwy, A.; Vincent-Mistiaen, Z.I.; Thompson, B.J. YAP and TAZ in Epithelial Stem Cells: A Sensor for Cell Polarity, Mechanical Forces and Tissue Damage. Bioessays 2016, 38, 644–653. [Google Scholar] [CrossRef] [Green Version]
- Mahoney, J.E.; Mori, M.; Szymaniak, A.D.; Varelas, X.; Cardoso, W.V. The Hippo Pathway Effector Yap Controls Patterning and Differentiation of Airway Epithelial Progenitors. Dev. Cell 2014, 30, 137–150. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Fallon, T.R.; Saladi, S.V.; Pardo-Saganta, A.; Villoria, J.; Mou, H.; Vinarsky, V.; Gonzalez-Celeiro, M.; Nunna, N.; Hariri, L.P.; et al. Yap Tunes Airway Epithelial Size and Architecture by Regulating the Identity, Maintenance, and Self-Renewal of Stem Cells. Dev. Cell 2014, 30, 151–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbediwy, A.; Vincent-Mistiaen, Z.I.; Spencer-Dene, B.; Stone, R.K.; Boeing, S.; Wculek, S.K.; Cordero, J.; Tan, E.H.; Ridgway, R.; Brunton, V.G.; et al. Integrin Signalling Regulates YAP and TAZ to Control Skin Homeostasis. Development 2016, 143, 1674–1687. [Google Scholar] [CrossRef] [Green Version]
- Gokey, J.J.; Sridharan, A.; Xu, Y.; Green, J.; Carraro, G.; Stripp, B.R.; Perl, A.-K.T.; Whitsett, J.A. Active Epithelial Hippo Signaling in Idiopathic Pulmonary Fibrosis. JCI Insight 2018. [Google Scholar] [CrossRef] [Green Version]
- Montaño-Machado, V.; Chevallier, P.; Mantovani, D.; Pauthe, E. On the Potential for Fibronectin/Phosphorylcholine Coatings on PTFE Substrates to Jointly Modulate Endothelial Cell Adhesion and Hemocompatibility Properties. Biomatter 2015, 5, e979679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bershadsky, A.D.; Balaban, N.Q.; Geiger, B. Adhesion-Dependent Cell Mechanosensitivity. Annu. Rev. Cell Dev. Biol. 2003, 19, 677–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiger, B.; Spatz, J.P.; Bershadsky, A.D. Environmental Sensing through Focal Adhesions. Nat. Rev. Mol. Cell Biol. 2009, 10, 21–33. [Google Scholar] [CrossRef]
- Gupta, M.; Doss, B.; Lim, C.T.; Voituriez, R.; Ladoux, B. Single Cell Rigidity Sensing: A Complex Relationship between Focal Adhesion Dynamics and Large-Scale Actin Cytoskeleton Remodeling. Cell Adhension Migr. 2016, 10, 554–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prager-Khoutorsky, M.; Lichtenstein, A.; Krishnan, R.; Rajendran, K.; Mayo, A.; Kam, Z.; Geiger, B.; Bershadsky, A.D. Fibroblast Polarization Is a Matrix-Rigidity-Dependent Process Controlled by Focal Adhesion Mechanosensing. Nat. Cell Biol. 2011, 13, 1457–1465. [Google Scholar] [CrossRef]
- Meng, Z.; Qiu, Y.; Lin, K.C.; Kumar, A.; Placone, J.K.; Fang, C.; Wang, K.-C.; Lu, S.; Pan, M.; Hong, A.W.; et al. RAP2 Mediates Mechanoresponses of the Hippo Pathway. Nature 2018, 560, 655–660. [Google Scholar] [CrossRef] [Green Version]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Elosegui-Artola, A.; Andreu, I.; Beedle, A.E.M.; Lezamiz, A.; Uroz, M.; Kosmalska, A.J.; Oria, R.; Kechagia, J.Z.; Rico-Lastres, P.; Le Roux, A.-L.; et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 2017, 171, 1397–1410.e14. [Google Scholar] [CrossRef]
- Totaro, A.; Panciera, T.; Piccolo, S. YAP/TAZ Upstream Signals and Downstream Responses. Nat. Cell Biol. 2018, 20, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Remuzzi, A.; Bonandrini, B.; Tironi, M.; Longaretti, L.; Figliuzzi, M.; Conti, S.; Zandrini, T.; Osellame, R.; Cerullo, G.; Raimondi, M.T. Effect of the 3D Artificial Nichoid on the Morphology and Mechanobiological Response of Mesenchymal Stem Cells Cultured In Vitro. Cell 2020, 9, 1873. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, S.; Saito, A.; Nagase, T. YAP/TAZ Signaling as a Molecular Link between Fibrosis and Cancer. Int J. Mol. Sci. 2018, 19, 3674. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Sun, Z.; Chen, T.; Pan, J.; Shen, Y.; Chen, X.; Zhou, X.; Cheng, R.; Yang, Y. The Role of MiR-431-5p in Regulating Pulmonary Surfactant Expression in Vitro. Cell. Mol. Biol. Lett. 2019, 24, 25. [Google Scholar] [CrossRef]
- Meyer, K.C. Diagnosis and Management of Interstitial Lung Disease. Transl. Respir. Med. 2014, 2, 4. [Google Scholar] [CrossRef] [Green Version]
- Weaver, T.E.; Na, C.-L.; Stahlman, M. Biogenesis of Lamellar Bodies, Lysosome-Related Organelles Involved in Storage and Secretion of Pulmonary Surfactant. Semin. Cell Dev. Biol. 2002, 13, 263–270. [Google Scholar] [CrossRef]
- Rose, F.; Kürth-Landwehr, C.; Sibelius, U.; Reuner, K.H.; Aktories, K.; Seeger, W.; Grimminger, F. Role of Actin Depolymerization in the Surfactant Secretory Response of Alveolar Epithelial Type II Cells. Am. J. Respir. Crit. Care Med. 1999, 159, 206–212. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campiglio, C.E.; Figliuzzi, M.; Silvani, S.; Tironi, M.; Conti, S.; Boschetti, F.; Remuzzi, A. Influence of Culture Substrates on Morphology and Function of Pulmonary Alveolar Cells In Vitro. Biomolecules 2021, 11, 675. https://doi.org/10.3390/biom11050675
Campiglio CE, Figliuzzi M, Silvani S, Tironi M, Conti S, Boschetti F, Remuzzi A. Influence of Culture Substrates on Morphology and Function of Pulmonary Alveolar Cells In Vitro. Biomolecules. 2021; 11(5):675. https://doi.org/10.3390/biom11050675
Chicago/Turabian StyleCampiglio, Chiara Emma, Marina Figliuzzi, Sara Silvani, Matteo Tironi, Sara Conti, Federica Boschetti, and Andrea Remuzzi. 2021. "Influence of Culture Substrates on Morphology and Function of Pulmonary Alveolar Cells In Vitro" Biomolecules 11, no. 5: 675. https://doi.org/10.3390/biom11050675