Neutrophil-to-Lymphocyte Ratio as a Cardiovascular Risk Marker May Be Less Efficient in Women Than in Men
Abstract
:1. Differences between Men and Women in Cardiovascular Disease Expression and Cardio-Metabolic Pathways
1.1. Differences between Men and Women in Cardiovascular Disease Expression
1.2. Differences between Men and Women in Cardio-Metabolic Pathways
2. Proposed Underlying Mechanisms of Differences between Men and Women in Cardiovascular Disease Patterns
2.1. The Effects of Variations of the Female Sex Hormones
2.2. The Effects of Sex Specific Differences in Immune Responses
2.3. The Effects of Age-Related Changes in Immune Mechanisms
3. Leukocyte Counts and Ratios as Markers of Inflammation in Cardiovascular Disease
4. Neutrophil-to-Lymphocyte Ratio (NLR) as a New Cardiovascular Risk Marker
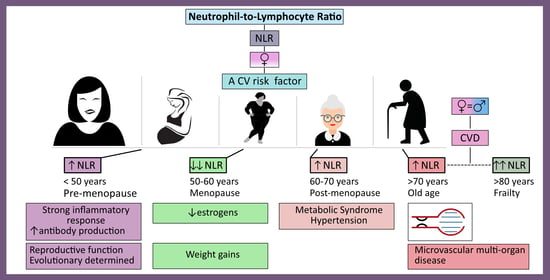
5. Sex Specific Differences in NLR across the Life Course–Associations with Immune Mechanisms Contributing to Sex Specific Differences in Patterns of CVD
5.1. Sex Specific Differences in NLR in the Age of Pre-Menopause
5.2. Sex Specific Differences in NLR in the Age around Menopause
5.3. Sex Specific Differences in NLR in the Age of Post-Menopause
6. A Summary of the Review
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, 9. [Google Scholar] [CrossRef] [PubMed]
- WHO Website. Aging and Health. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 31 January 2021).
- Graham, I.M. The importance of total cardiovascular risk assessment in clinical practice. Eur. J. Gen. Pract. 2006, 12, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Mulvagh, S.L.; Merz, C.N.; Buring, J.E.; Manson, J.E. Cardiovascular Disease in Women: Clinical Perspectives. Circ. Res. 2016, 118, 1273–1293. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, A.; Scovelle, A.J.; Milner, A.J.; Kavanagh, A. Gender/Sex as a Social Determinant of Cardiovascular Risk. Circulation 2018, 137, 854–864. [Google Scholar] [CrossRef]
- Prentice, A.M. The emerging epidemic of obesity in developing countries. Int. J. Epidemiol. 2005, 35, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, H.R.; Srichai, M.B.; Iqbal, S.N.; Slater, J.N.; Mancini, G.B.J.; Feit, F.; Pena-Sing, I.; Axel, L.; Attubato, M.J.; Yatskar, L.; et al. Mechanisms of Myocardial Infarction in Women Without Angiographically Obstructive Coronary Artery Disease. Circulation 2011, 124, 1414–1425. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V. Sex and gender differences in symptoms of myocardial ischaemia. Eur. Hear. J. 2011, 32, 3064–3066. [Google Scholar] [CrossRef]
- Albadri, A.; Lai, K.; Wei, J.; Landes, S.; Mehta, P.K.; Li, Q.; Johnson, D.; Reis, S.E.; Kelsey, S.F.; Bittner, V.; et al. Inflammatory biomarkers as predictors of heart failure in women without obstructive coronary artery disease: A report from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE). PLoS ONE 2017, 12, e0177684. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome-a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, H.; Lehmkuhl, E.; Weickert, M.O. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin. Res. Cardiol. 2006, 95, 136–147. [Google Scholar] [CrossRef]
- Šabanović, Š.; Majnarić Trtica, L.J.; Babič, F.; Vadovsky, M.; Paralič, J.; Včev, A.; Holzinger, A. Metabolic syndrome in hypertensive women in the age of menopause: A case study on data from general practice electronic health records. BMC Med. Inform. Decis. Mak. 2018, 18, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vryonidou, A.; Paschou, S.A.; Muscogiuri, G.; Orio, F.; Goulis, D.G. Mechanisms in Endocrinology: Metabolic syndrome through the female life cycle. Eur. J. Endocrinol. 2015, 173, R153–R163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, K.A.; Crawford, S.L.; Chae, C.U.; Everson-Rose, S.A.; Sowers, M.F.R.; Sternfeld, B.; Sutton-Tyrrell, K. Are Changes in Cardiovascular Disease Risk Factors in Midlife Women Due to Chronological Aging or to the Menopausal Transition? J. Am. Coll. Cardiol. 2009, 54, 2366–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancuso, P.; Bouchard, B. The Impact of Aging on Adipose Function and Adipokine Synthesis. Front. Endocrinol. 2019, 10, 137. [Google Scholar] [CrossRef] [Green Version]
- Majnarić, L.T.; Martinović, I.; Šabanović, Š.; Rudan, S.; Babič, F.; Wittlinger, T. The Effect of Hypertension Duration and the Age of Onset on CV Risk Factors Expression in Perimenopausal Women. Int. J. Hypertens. 2019, 2019, 9848125. [Google Scholar] [CrossRef]
- Davis, S.R.; Castelo-Branco, C.; Chedraui, P.; Lumsden, M.A.; Nappi, R.E.; Shah, D.; Villaseca, P. Understanding weight gain at menopause. Climacteric 2012, 15, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Thor, D.; Zhang, R.; Anderson, L.; Bose, D.D.; Dubé, G.P.; Rahimian, R. Effects of 17 β-estradiol on lipopolysacharride-induced intracellular adhesion molecule-1 mRNA expression and Ca2+ homeostasis alteration in human endothelial cells. Vasc. Pharmacol. 2010, 53, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Quintero, O.L.; Amador-Patarroyo, M.J.; Montoya-Ortiz, G.; Rojas-Villarraga, A.; Anaya, J.-M. Autoimmune disease and gender: Plausible mechanisms for the female predominance of autoimmunity. J. Autoimmun. 2012, 38, J109–J119. [Google Scholar] [CrossRef]
- Pennell, L.M.; Galligan, C.L.; Fish, E.N. Sex affects immunity. J. Autoimmun. 2012, 38, J282–J291. [Google Scholar] [CrossRef]
- Fairweather, D. Sex Differences in Inflammation during Atherosclerosis. Clin. Med. Insights Cardiol. 2014, 8 (Suppl. 3), CMC.S17068-59. [Google Scholar] [CrossRef] [Green Version]
- Vandenbroeck, K. Cytokine gene polymorphisms and human autoimmune disease in the era of genome-wide association studies. J. Interferon Cytokine Res. 2012, 32, 139–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwakura, Y.; Ishigame, H. The IL-23/IL-17 axis in inflammation. J. Clin. Investig. 2006, 116, 1218–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuda, S.; Nakashima, A.; Shima, T.; Saito, S. New Paradigm in the Role of Regulatory T Cells During Pregnancy. Front. Immunol. 2019, 10, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinewietfeld, M.; Hafler, D.A. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin. Immunol. 2013, 25, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Gianturco, L.; Bodini, B.D.; Atzeni, F.; Colombo, C.; Stella, D.; Sarzi-Puttini, P.; Drago, L.; Galaverna, S.; Turiel, M. Cardio-vascular and autoimmune diseases in females: The role of microvasculature and dysfunctional endotheli-um. Atherosclerosis 2015, 241, 259–263. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Delzell, E.; Muntner, P.; Hillegass, W.B.; Safford, M.M.; Millan, I.Y.; Crowson, C.S.; Curtis, J.R. The asso-ciation between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2014, 73, 1301–1308. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Bupp, M.R.G. Sex, the aging immune system, and chronic disease. Cell. Immunol. 2015, 294, 102–110. [Google Scholar] [CrossRef]
- Ma, S.; Wang, C.; Mao, X.; Hao, Y. B Cell Dysfunction Associated with Aging and Autoimmune Diseases. Front. Immunol. 2019, 10, 318. [Google Scholar] [CrossRef] [Green Version]
- Vadasz, Z.; Haj, T.; Kessel, A.; Toubi, E. Age-related autoimmunity. BMC Med. 2013, 11, 94. [Google Scholar] [CrossRef]
- Ley, K.; Smith, E.; Stark, M.A. IL-17A-producing neutrophil-regulatory Tn lymphocytes. Immunol. Res. 2006, 34, 229–242. [Google Scholar] [CrossRef]
- Araos, P.; Figueroa, S.; Amador, C.A. The Role of Neutrophils in Hypertension. Int. J. Mol. Sci. 2020, 21, 8536. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Dejana, E.; Fiocchi, C. Immune regulation by microvascular endothelial cells: Directing innate and adaptive im-munity, coagulation, and inflammation. J. Immunol. 2007, 178, 6017–6022. [Google Scholar] [CrossRef] [PubMed]
- Mortaz, E.; Alipoor, S.D.; Adcock, I.M.; Mumby, S.; Koenderman, L. Update on neutrophil function in severe inflamma-tion. Front. Immunol. 2018, 9, 2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frangogiannis, N. Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef] [PubMed]
- Wick, G.; Berger, P.; Jansen-Dürr, P.; Grubeck-Loebenstein, B. A Darwinian-evolutionary concept of age-related diseases. Exp. Gerontol. 2003, 38, 13–25. [Google Scholar] [CrossRef]
- Climie, R.E.; Van Sloten, T.T.; Bruno, R.-M.; Taddei, S.; Empana, J.-P.; Stehouwer, C.D.; Sharman, J.E.; Boutouyrie, P.; Laurent, S. Macrovasculature and Microvasculature at the Crossroads Between Type 2 Diabetes Mellitus and Hypertension. Hypertension 2019, 73, 1138–1149. [Google Scholar] [CrossRef]
- Lockhart, C.J.; Hamilton, P.K.; Quinn, C.E.; McVeigh, G.E. End-organ dysfunction and cardiovascular outcomes: The role of the microcirculation. Clin. Sci 2009, 116, 175–190. [Google Scholar] [CrossRef] [Green Version]
- Kaptoge, S.; Di Angelantonio, E.; Lowe, G.; Pepys, M.B.; Thompson, S.G.; Collins, R.; Danesh, J. C-reactive protein concentra-tion and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 2010, 375, 132–140. [Google Scholar]
- Dale, D.C.; Boxer, L.; Liles, W.C. The phagocytes: Neutrophils and monocytes. Blood 2008, 112, 935–945. [Google Scholar] [CrossRef]
- Rathod, K.S.; Kapil, V.; Velmurugan, S.; Khambata, R.S.; Siddique, U.; Khan, S.; Van Eijl, S.; Gee, L.C.; Bansal, J.; Pitrola, K.; et al. Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans. J. Clin. Investig. 2017, 127, 169–182. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Lymphocytes and the cellular basis of adaptive immunity. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Arce-Sillas, A.; Álvarez-Luquín, D.D.; Tamaya-Domínguez, B.; Gomez-Fuentes, S.; Trejo-García, A.; Melo-Salas, M.; Cárdenas, G.; Rodríguez-Ramírez, J.; Adalid-Peralta, L. Regulatory T Cells: Molecular Actions on Effector Cells in Immune Regulation. J. Immunol. Res. 2016, 2016, 1720827. [Google Scholar] [CrossRef] [Green Version]
- Sesti-Costa, R.; De Moraes-Vieira, P.M.M.; Cervantes-Barragan, L. Dendritic Cells: Immune Response in Infectious Diseases and Autoimmunity. Mediat. Inflamm. 2020, 2020, 2948525. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Weiss, S.T. Host and environmental factors influencing the peripheral blood leukocyte count. Am. J. Epidemiol. 1991, 134, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Shen, H.; Wang, G.; Zhang, P.; Liu, Q.; Du, J. Prognostic Significance of Systemic Inflammation-Based Lymphocyte- Monocyte Ratio in Patients with Lung Cancer: Based on a Large Cohort Study. PLoS ONE 2014, 9, e108062. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.; Tel, S.; Rija, J.; Bhat, H.; Raza, M. Neutrophil to lymphocyte ratio and cardiovascular diseases: A review. Expert Rev. Cardiovasc. Ther. 2013, 11, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Ambrosy, A.P.; Greene, S.J.; Mentz, R.J.; Subacius, H.P.; Maggioni, A.P.; Swedberg, K.; Nodari, S.; Zan-nad, F.; Konstam, M.A.; et al. EVEREST trial investigators. Predictive value of low relative lymphocyte count in patients hospitalized for heart failure with reduced ejection fraction: Insights from the EVEREST trial. Circ. Heart Fail. 2012, 5, 750–758. [Google Scholar] [CrossRef] [Green Version]
- Angkananard, T.; Anothaisintawee, T.; McEvoy, M.; Attia, J.; Thakkinstian, A. Neutrophil lymphocyte ratio and cardiovascu-lar disease risk: A systematic review and meta-analysis. BioMed Res. Int. 2018, 2018, 2703518. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, T.Z.; Lee, M.-S. Carotid Intima-Media Thickness and Plaque in Cardiovascular Risk Assessment. JACC: Cardiovasc. Imaging 2014, 7, 1025–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corriere, T.; Di Marca, S.; Cataudella, E.; Pulvirenti, A.; Alaimo, S.; Stancanelli, B.; Malatino, L. Neutrophil-to-Lymphocyte Ratio is a strong predictor of atherosclerotic carotid plaques in older adults. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gu, C.; Wang, F.; Lv, B.; Zhang, C.; Peng, R.; Cong, X.; Chen, X. Association of Neutrophil–Lymphocyte Ratio and the Presence of Noncalcified or Mixed Coronary Atherosclerotic Plaques. Angiology 2018, 69, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.; Ertaş, F.; İslamoğlu, Y.; Kaya, Z.; Atılgan, Z.A. Association between neutrophil to lymphocyte ratio and severity of coronary artery disease. Clin. Appl. Thromb./Hemost. 2014, 20, 50–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalay, N.; Dogdu, O.; Koc, F.; Yarlıoglues, M.; Ardic, I.; Akpek, M.; Cicek, D.; Oguzhan, A.; Ergin, A.; Kaya, M.G. Hematologic Parameters and Angiographic Progression of Coronary Atherosclerosis. Angiology 2011, 63, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Erturk, M.; Cakmak, H.A.; Surgit, O.; Celik, O.; Aksu, H.U.; Akgul, O.; Gurdogan, M.; Bulut, U.; Ozalp, B.; Akbay, E.; et al. The predictive value of elevated neutrophil to lymphocyte ratio for long-term cardiovascular mortality in peripheral arterial occlusive disease. J. Cardiol. 2014, 64, 371–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonyali, S.; Ceylan, C.; Yahsi, S.; Karakan, M.S. Does neutrophil to lymphocyte ratio demonstrate deterioration in renal function? Ren. Fail. 2018, 40, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Zazula, A.D.; Précoma-Neto, D.; Gomes, A.M.; Kruklis, H.; Barbieri, G.F. An assessment of neutrophils/lymphocytes ratio in patients suspected of acute coronary syndrome. Arq. Bras. Cardiol. 2008, 90, 31. [Google Scholar]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [Green Version]
- Demir, M. The Relationship Between Neutrophil Lymphocyte Ratio and Non-dipper Hypertension. Clin. Exp. Hypertens. 2013, 35, 570–573. [Google Scholar] [CrossRef]
- Buyukkaya, E.; Karakaş, M.F.; Karakaş, E.; Akçay, A.B.; Tanboga, I.H.; Kurt, M.; Sen, N. Correlation of Neutrophil to Lymphocyte Ratio with the Presence and Severity of Metabolic Syndrome. Clin. Appl. Thromb. 2014, 20, 159–163. [Google Scholar] [CrossRef]
- Bahadir, A.; Baltaci, D.; Turker, Y.; Iliev, D.; Ozturk, S.; Deler, M.H.; Sariguzel, Y.C. Is the neutrophil-to-lymphocyte ratio indicative of inflammatory state in patients with obesity and metabolic syndrome? Anatol. J. Cardiol. 2015, 15, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Babio, N.; Ibarrola-Jurado, N.; Bulló, M.; Martínez-González, M.Á.; Wärnberg, J.; Salaverría, I.; Ortega-Calvo, M.; Estruch, R.; Serra-Majem, L.; Covas, M.I.; et al. White Blood Cell Counts as Risk Markers of Developing Metabolic Syndrome and Its Components in the Predimed Study. PLoS ONE 2013, 8, e58354. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Wang, Y.; Fang, S.; Chen, Y.; Zhang, W.; Xia, F.; Wang, N.; Lu, Y. Associations between the Neutrophil-to-Lymphocyte Ratio and Diabetic Complications in Adults with Diabetes: A Cross-Sectional Study. J. Diabetes Res. 2020, 2020, 6219545. [Google Scholar] [CrossRef]
- Ardahanli, I.; Celik, M.; Takir, M. Relationship between Neutrophil/Lymphocyte Ratio and Cardiometabolic Values in Patients with Prediabetes. Glob. J. Endocrinol. Metab. 2020, 2, 1–4. [Google Scholar] [CrossRef]
- Howard, R.; Scheiner, A.; Kanetsky, P.A.; Egan, K.M. Sociodemographic and lifestyle factors associated with the neutro-phil-to-lymphocyte ratio. Ann. Epidemiol. 2019, 38, 11–21.e6. [Google Scholar] [CrossRef]
- Fest, J.; Ruiter, R.; Ikram, M.A.; Voortman, T.; van Eijck, C.H.J.; Stricker, B.H. Reference values for white blood-cell-based in-flammatory markers in the Rotterdam Study: A population-based prospective cohort study. Sci. Rep. 2018, 8, 10566. [Google Scholar] [CrossRef]
- Davis, J.L.; Moutinho, V., Jr.; Panageas, K.S.; Coit, D.G. A peripheral blood biomarker estimates probability of survival: The neutrophil-lymphocyte ratio in noncancer patients. Biomark. Med. 2016, 10, 953–957. [Google Scholar] [CrossRef] [Green Version]
- Cramer, D.W.; Vitonis, A.F. Signatures of reproductive events on blood counts and biomarkers of inflammation: Implications for chronic disease risk. PLoS ONE 2017, 12, e0172530. [Google Scholar] [CrossRef]
- Wu, L.; Zou, S.; Wang, C.; Tan, X.; Yu, M. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in Chinese Han popula-tion from Chaoshan region in South China. BMC Cardiovasc. Disord. 2019, 19, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Kim, N.Y.; Na, S.H.; Youn, Y.H.; Shin, C.S. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine 2018, 97, e11138. [Google Scholar] [CrossRef]
- Azab, B.; Camacho-Rivera, M.; Taioli, E. Average Values and Racial Differences of Neutrophil Lymphocyte Ratio among a Nationally Representative Sample of United States Subjects. PLoS ONE 2014, 9, e112361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engler, H.; Benson, S.; Wegner, A.; Spreitzer, I.; Schedlowski, M.; Elsenbruch, S. Men and women differ in inflammatory and neuroendocrine responses to endotoxin but not in the severity of sickness symptoms. Brain Behav. Immun. 2016, 52, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Lansky, A.J.; Ng, V.G.; Maehara, A.; Weisz, G.; Lerman, A.; Mintz, G.S.; De Bruyne, B.; Farhat, N.; Niess, G.; Jankovic, I.; et al. Gender and the Extent of Coronary Atherosclerosis, Plaque Composition, and Clinical Outcomes in Acute Coronary Syndromes. JACC Cardiovasc. Imaging 2012, 5, S62–S72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.; Steffes, M.; Lee, D.H.; Himes, J.H.; Jacobs, D.R., Jr. Association of inflammation with worsening HOMA-insulin re-sistance. Diabetologia 2009, 52, 2337–2344. [Google Scholar] [CrossRef] [Green Version]
- Stark, M.A.; Huo, Y.; Burcin, T.L.; Morris, M.A.; Olson, T.S.; Ley, K. Phagocytosis of apoptotic neutrophils regulates granulo-poiesis via IL-23 and IL-17. Immunity 2005, 22, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Samson, L.D.; Boots, A.M.H.; Ferreira, J.A. In-depth immune cellular profiling reveals sex-specific associations with frailty. Immun. Ageing 2020, 17, 20. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Frisoli, A., Jr.; Ingham, S.J.; Paes, Â.T.; Tinoco, E.; Greco, A.; Zanata, N.; Pintarelli, V.; Elber, I.; Borges, J.; Camargo Carvalho, A.C. Frailty predictors and outcomes among older patients with cardiovascular disease: Data from Fragicor. Arch. Gerontol. Geriatr. 2015, 61, 1–7. [Google Scholar] [CrossRef]
- Ahmadi, S.-F.; Streja, E.; Zahmatkesh, G.; Streja, D.; Kashyap, M.; Moradi, H.; Molnar, M.Z.; Reddy, U.; Amin, A.N.; Kovesdy, C.P.; et al. Reverse Epidemiology of Traditional Cardiovascular Risk Factors in the Geriatric Population. J. Am. Med. Dir. Assoc. 2015, 16, 933–939. [Google Scholar] [CrossRef] [Green Version]
- Gordon, E.H.; Hubbard, R.E. Do sex differences in chronic disease underpin the sex-frailty paradox? Mech. Ageing Dev. 2019, 179, 44–50. [Google Scholar] [CrossRef]


| Authors | Findings |
|---|---|
| Corriere et al. [53] | Demonstrated that NLR is a strong predictor of the presence and number of carotid atherosclerotic plaques |
| Li et al. [54] | Demonstrated an association between NLR and mixed and non-calcified plaques in the coronary arteries of patients with chest pain |
| Kaya et al. [55] | Found significantly higher NLR values in patients with severe coronary atherosclerosis |
| Kalay et al. [56] | Demonstrated that NLR predicts coronary atherosclerosis progression and suggested it as a marker for monitoring |
| Erturk et al. [57] | NLR values higher than 3.0 were predict CV mortality in patients with peripheral arterial occlusive disease |
| Tonyali et al. [58] | NLR values equal to or higher than 2.5 were shown to predict severe atherosclerosis with a sensitivity and specificity of 62% and 69%, respectively |
| Zazula et al. [59] | Found that patients with chest pain that was not caused by cardiac disease, had NLR values of 3.0 ± 1.6, those with chest pain caused by unstable angina had NLR values of 3.6 ± 2.9, and those with MI had much higher values—4.8 ± 3.7 with non-STEMI, and 6.9 ± 5.7 with STEMI, and concluded that NLR value above 5.7 had 91% specificity for the diagnosis of ACS. |
| Authors | Findings |
|---|---|
| Demir et al. [61] | Increased NLR was also found in patients with hypertension of the sort called “non-dipper hypertension”, which does not show a circadian rhythm and is connected with an increased risk of CV events, as a consequence of microvascular changes |
| Tonyali et al. [58] | Demonstrated in patients with partial or complete nephrectomy, that NLR can represent renal function and renal reserve, making it a good marker of declining renal function |
| Buyukkaya et al. [62] | Found that increased values for neutrophils and NLR, with an optimal NLR threshold of 1.84, correlate with the severity of MS, without a significant change in the lymphocyte count |
| Bahadir et al. [63] | Did not find NLR to be a good predictor of inflammation severity in obese patients with MS and without DM2 but indicated that a more significant role was played by CRP and lymphocyte count |
| Babio et al. [64] | Higher baseline neutrophil counts and an increase in neutrophil counts during follow-up, were both independently associated with a risk of MS in people of 55 years or above, and free of CVD. Although these associations were also seen with total WBC and some other leucocyte subpopulations, neutrophils showed the strongest and most consistent associations, in particular when predicting dyslipidemia associated with MS |
| Wan et al. [65] | Higher baseline neutrophil counts and an increase in neutrophil counts during follow-up, were both independently associated with a risk of MS in people of 55 years or above, and free of CVD. Although these associations were also seen with total WBC and some other leucocyte subpopulations, neutrophils showed the strongest and most consistent associations, in particular when predicting dyslipidemia associated with MS |
| Ardahanli et al. [66] | Detected increased NLR values in pre-diabetic patients, compared to healthy controls, suggesting its suitability as a screening marker |
| Howard et al. [67] | Multiple demographic and lifestyle factors are associated with NLR, independent of important comorbidities, including heart disease, cancer, diabetes, and hypertension |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trtica Majnarić, L.; Guljaš, S.; Bosnić, Z.; Šerić, V.; Wittlinger, T. Neutrophil-to-Lymphocyte Ratio as a Cardiovascular Risk Marker May Be Less Efficient in Women Than in Men. Biomolecules 2021, 11, 528. https://doi.org/10.3390/biom11040528
Trtica Majnarić L, Guljaš S, Bosnić Z, Šerić V, Wittlinger T. Neutrophil-to-Lymphocyte Ratio as a Cardiovascular Risk Marker May Be Less Efficient in Women Than in Men. Biomolecules. 2021; 11(4):528. https://doi.org/10.3390/biom11040528
Chicago/Turabian StyleTrtica Majnarić, Ljiljana, Silva Guljaš, Zvonimir Bosnić, Vatroslav Šerić, and Thomas Wittlinger. 2021. "Neutrophil-to-Lymphocyte Ratio as a Cardiovascular Risk Marker May Be Less Efficient in Women Than in Men" Biomolecules 11, no. 4: 528. https://doi.org/10.3390/biom11040528