1. Introduction
To maintain food security and crop productivity as well as to maintain sustainable agriculture, the accumulation of heavy metals on agricultural lands must be restricted. Crop plants must be qualified to cope with the adverse effects of heavy metals using novel strategies to minimize plant uptake or increase the plant’s resistance to their massive damage. Farmland contaminated with heavy metals is causing a dangerous decline in the efficiency of processes in plants concerning growth and productivity [
1,
2,
3,
4,
5,
6,
7,
8]. The increase in heavy metals in the soil stimulates oxidative stress linked to the overproduction of ROSs (reactive species of oxygen radicals). ROSs threaten plants by endangering various pathways related to both physiobiochemistry and molecular biology [
8,
9,
10]. The decrease in plant growth due to heavy metals depends on some factors, including plant species, heavy metal concentration, growth conditions, and experimental conditions [
11,
12]. Among various heavy metals, the highest toxic effect of cadmium (Cd)-related pollution has been observed in wheat [
1,
3,
6,
10], pea [
13], and rice [
8,
14].
Agricultural land and the plants cultivated on it have become severely threatened due to Cd toxicity worldwide [
4,
15]. Even with low concentrations, Cd is harmful to crop plants, and thus harmful to humans and animals that feed on these Cd-contaminated plants. After being absorbed by the root system of the plant, Cd easily transports into the shoot and adversely influences plant morphology and physiobiochemistry during all stages of the plant life cycle (e.g., germination, vegetative growth, and fruiting stages) [
16]. The usual symptoms common to plants are stunted root and shoot, chlorosis of leaves, and a sharp decrease in biomass accumulation, all of which ultimately lead to plant death [
9,
10,
11,
12,
13,
14,
15,
16]. Cd accumulation greatly affects the absorption and transportation of almost all key nutrients in different parts of the plant [
17,
18,
19]. These adverse events, particularly concerning the interference between the Cd metal and essential nutrients, can be attributed to the channel competition for nutrient uptake occurring at the molecular level [
18].
Often, the endogenous antioxidant defense system is not sufficient for the plant to defend against environmental foes, including Cd. Thus, a plant extract known to be a biostimulant, such as maize grain extract (MEg), can be used as a foliar spray and/or seed priming solution to support plants to increase their tolerance to environmental opponents [
3,
20,
21,
22,
23], including Cd stress [
3].
Presently, MEg has been used to enhance plant efficiency under different stress conditions, including Cd stress, as it is an essential organic biostimulator rich in many growth-promoting substances for different stressed plants, such as antioxidants, phytohormones, and essential nutrients [
3]. After applying MEg, plant morphology, physiology, and biochemistry have been positively modified along with stimulation of plant tolerance against damage of some stresses [
3,
20,
21,
22,
23]. Therefore, MEg is a potent novel biostimulator to give stressed plants the power to resist damage from environmental opponents.
Among the most essential crops around the world, maize (
Zea mays L.) is ranked third after wheat and rice. In developing countries,
Zea mays is one of the key dietary food components because of its high nutritional value [
24]. Due to the increasing environmental opponents caused by climate change, and the rapid and turbulent growth of industries and demographics, the yield of
Zea mays is decreasing worldwide, and the issue has been exacerbated by the arrival of Cd in humans and animals [
25], thus toxicity from Cd in
Zea mays is a major concern.
As found in the preliminary study of the current investigation (
Table S1),
Zea mays (as a C
4 crop) is more sensitive to Cd and more responsive to MEg than other species such as wheat [
3], so it was selected for the present study. There are no investigations on the influences of MEg and Cd on
Zea mays; however, only one paper has been published dealing with the influences of MEg and Cd, but it focused on
Triticum aestivum [
3]. To date, no investigations have been conducted with silymarin (Sm)-enriched MEg (MEg+Sm) for
Zea mays grown under Cd stress. Therefore, this is the first investigation in which MEg+Sm was applied to leaves to encourage the growth of
Zea mays under Cd stress. MEg lacks silymarin, so MEg was enriched with silymarin for this study.
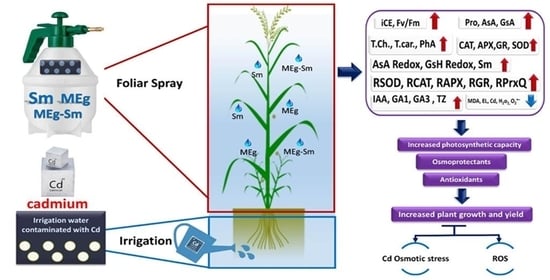
This investigation was, therefore, aimed at studying the influences of MEg+Sm on plant growth, physiobiochemistry, enzyme activities, and enzyme-related gene expressions in Cd-stressed Zea mays. To fulfill this hypothesis, a set of morphophysiobiochemical and molecular indices was identified to investigate MEg+Sm-induced stress tolerance to Cd in Zea mays.
4. Discussion
No information is available on the foliar application of maize plants with a maize grain extract (MEg) enriched with silymarin (Sm); MEg-Sm to attenuate the adverse impacts of cadmium (Cd) stress on plant performance, which responded positively to the novel MEg-Sm. Recently, some articles have been reported positive changes in plant growth, biochemical attributes, and plant defensive system (e.g., antioxidative enzymes and nonenzymatic antioxidants) after treating plants with MEg alone under some stresses [
3,
20,
21,
22,
23], indicating the importance of MEg. However, the current study provides impressive results such as a considerable increase in maize plant performance and defense system along with gene expression related to antioxidant enzymes due to the application of MEg-Sm, which outperformed MEg alone due to that Sm increased the efficiency of the extract.
Presently, sustainable maize returns challenge various issues such as reduced soil fertility and increased degradation due to contamination of farmland with heavy metals, including Cd. Cd stress is one of the key concerns facing maize production, restricting crop yield. Cd frequently causes disturbances of various morphophysiobiochemical and molecular features. It restricts plant growth, disrupts chlorophyll biosynthesis, and thus photosynthesis, and negatively affects the defense system and antioxidant gene expression in plants [
8,
14]. Therefore, attenuating the toxic effect of Cd on plant growth and biochemical processes remains a constant concern of scientists. There is an urgent need to ameliorate Cd tolerance in maize through sustainable and ecofriendly strategies, which are the key to achieving security for more foods for an ever-expanding population. In the present study, 0.5 mM Cd severely decreased maize plant growth traits (e.g., plant height, leaves number, leaves area per plant, fresh and dry weight of shoot system), photosynthetic efficiency (instantaneous carboxylation efficiency; iCE, pigment contents,
Fv/
Fm, and photochemical activity) and hormonal contents (
Figure 1 and
Figure 4,
Table 6). These negative results coincided with a harmful increase in markers of oxidative stress levels (O
2•− and H
2O
2), lipid peroxidation (MDA), ionic leakage (EL), and accumulation of Cd (
Figure 2). All of these negative results encouraged an increase in the plant’s enzymatic and nonenzymatic antioxidative defense system and Sm content (
Figure 3 and
Figure 4,
Table 6), and transcriptional level of genes related to enzymatic antioxidants (
Figure 5,
Table 6) to enable plants to cope with markers of oxidative stress overproduced by Cd stress.
The number of green leaves obtained from the tallest plants should be optimized; thus, green leaf area is a pivotal strategy for increasing photosynthesis efficiency and increasing dry matter output. Under normal or Cd stress conditions, foliar treating maize plants with MEg or Sm led to a significant rise in plant height, leaves number, and leaves area, which reflected positively on plant weight, especially dry matter output (
Figure 1,
Table 6). Treatment with Sm-enriched MEg (MEg-Sm) outperformed either MEg or Sm alone. This may be due to the improving effect of Sm, which has added to the various benefits of MEg. In addition to having a high antioxidative activity (89.22%), MEg contains several stimulating mechanisms such as antioxidants (proline, ascorbate; AsA, and glutathione; GSH) and various phytohormones (IAA, GA
1, GA
3, cytokinins including trans-zeatin, and salicylic acid; SA) (
Table 5). The increase in plant height and number of leaves resulting from the application of MEg-Sm contributed to an increase in plant leaf area, accompanied by an increase in photosynthetic pigment contents, all of which contributed to an increase in photosynthetic efficiency (
Fv/
Fm and photochemical activity). These positive results were positively reflected in dry matter accumulation (
Figure 1,
Table 6). All these positive results were achieved by MEg-Sm due to the minimized levels of Cd and markers of oxidative stress, which contributed to the reduction of MDA and EL (
Figure 2,
Table 6).
The enhanced effect of MEg-Sm (which outperformed the enhanced effect of MEg or Sm) on transcriptional gene levels related to antioxidant enzymes (SOD, CAT, APX, CR, and PrxQ) efficiently contributed to increasing levels and activities of antioxidant enzymes (
Figure 4 and
Figure 5,
Table 6), which in turn contributed to the improvement of hormonal homeostasis (
Figure 4,
Table 6). The significant improvement in the antioxidant defense state of maize plants by MEg-Sm treatment contributed to the minimization of Cd
2+ ions (
Figure 2,
Table 6), which resulted in the plants recovering from stress due to ROS suppression. This may enable plants to stabilize and balance their hormones so that they perform well.
It has been shown that antioxidants and hormonal homeostasis regulate plant growth and their physiobiochemical performance under Cd stress [
6,
23,
49]. As shown in
Table 5, MEg is rich in antioxidants and phytohormones, which are important mechanisms for improving growth, dry matter production, and physiobiochemical attributes, as well as the antioxidant defense system of maize plants grown under Cd stress (
Figure 1,
Figure 2,
Figure 3,
Figure 4 and
Figure 5,
Table 6). As a crucial mechanism that helped maize plants withstand Cd stress, increases in AsA and GSH contents and redox states were noticed (
Figure 3,
Table 6). An increase in hormonal content and homeostasis was also observed as another effective mechanism that enabled plants to withstand the effects of Cd stress (
Figure 4,
Table 6).
The major mechanism in this regard, the observed increases in transcriptional levels of genes related to the examined antioxidant enzymes, had a major role in withstanding the adverse effects of stress [
8,
50] in maize plants. In addition to these key mechanisms, increases in proline and Sm contents (
Figure 3,
Table 6) likely contributed to the increased defenses of the maize plant against Cd stress. Since many plant growth stimuli (e.g., AsA, GSH, IAA, GA
1, GA
3, cytokinins including trans-zeatin, SA, proline, and Sm) are present in MEg-Sm (
Table 5), which was the best treatment, it is considered a potent biostimulator to grow maize plants effectively under Cd stress (
Figure 1,
Figure 2,
Figure 3,
Figure 4 and
Figure 5,
Table 6).
Among the major growth stimulators present in MEg-Sm, proline, AsA, and GSH contents were greatly increased in Cd-stressed plants and contributed to stabilizing and maintaining cell membranes against stress damage [
2,
5,
51,
52,
53,
54,
55,
56,
57,
58,
59]. These positive effects of these antioxidants might be attributed to their roles in minimizing the Cd content or other toxic elements and the levels of oxidative stress markers (O
2•− and H
2O
2;
Figure 2,
Table 6), thus minimizing lipid peroxidation in cell membranes. As a result of all these positive results, the photosynthetic efficiency including
Fv/
Fm and iCE was maintained (
Figure 1,
Table 6) due to the integrity of cellular water content [
2,
52,
53,
55]. These positive results examined in this study were positively reflected in maize plant growth and dry matter accumulation (
Figure 1,
Table 6). Our findings are consistent with those in [
2,
5,
51,
52,
53,
54,
55,
56,
57,
58,
59]. Restoration of growth and dry matter accumulation of Cd-stressed maize plants are also due to the positive impacts of proline, AsA, and GSH present in MEg-Sm on increasing hormones (e.g., IAA, GA
1, GA
3, cytokinins including trans-zeatin, SA) content and homeostasis, which are necessary to restore the developmental growth of stressed plants [
20,
23,
60,
61,
62].
Phytohormones play a large role in signaling, biochemistry, and defense pathways in plants, providing a key mechanism for relieving heavy metal stress [
23,
63]. The increased growth of Cd-stressed maize plants was likely related to the increased partitioning of photosynthesized substances with plant development and phytohormone levels (
Figure 4,
Table 6). Phytohormones regulate membrane permeability, enzyme activity, secondary metabolism, plant growth, and plant reproduction [
23,
64]. Elevated gibberellins (GAs) are shown in stressed plants to enhance stress tolerance by enhancing gene expression [
20,
65,
66]. Thereafter, hormonal homeostasis under Cd stress could be a possible mechanism of GAs (e.g., GA
1 and GA
3) that stimulate Cd stress tolerance in the plant [
65,
66]. Cytokinins, including trans-Zeatin, and SA act to withstand stress. They play many regulatory roles in promoting plant growth, protein biosynthesis, and secondary metabolism [
61,
62,
66,
67]. They generally eliminate ROSs and increase the antigenicity of ABA under stress [
67,
68]. Another key phytohormone, IAA influences Cd toxicity, which has been relied upon to regulate many antioxidative activities, including the AsA-GSH cycle [
68,
69]. Altogether, phytohormones can raise antioxidant levels to reduce ROSs (e.g., O
2•− and H
2O
2) levels, helping reduce lipid peroxidation (MDA) to keep healthy plant growth [
49]. Phytohormones eliminate stressors’ impacts and promote rates of survival [
70,
71] by either enhancing shoot growth or regulating processes to prohibit plant growth (e.g., dormancy, withdrawal, and aging), and thus controlling growth activities in plants [
72,
73]. In our investigation, elevated hormonal content by MEg, Sm, or MEg-Sm (with a high preference for MEg-Sm) (
Figure 4,
Table 6) was associated with higher activity of the antioxidative defense system, antioxidant gene expression (
Figure 3,
Figure 4 and
Figure 5 and
Table 6) and suppression in ROSs; O
2•− and H
2O
2 levels, which minimized MDA, EL, and Cd levels and maximized plant performance and photosynthesis efficiency (
Figure 1 and
Figure 2,
Table 6).
The induced activity of antioxidant enzymes that are premium biochemical signals of stress can eliminate O
2•− and H
2O
2 stimulated by Cd. Despite elevated enzyme activity under Cd stress, spraying maize plants with MEg, Sm, or MEg-Sm (with a high preference for MEg-Sm) further elevated SOD, CAT, APX, and GR activity while stimulating increased transcriptional levels of the antioxidant enzyme genes (
Figure 4 and
Figure 5,
Table 6). In this study, promotion of antioxidant gene transcript levels was associated with increased levels of proline, AsA, and GSH in contributing to increased enzymatic activities as a protective mechanism for suppressing many types of ROSs like H
2O
2,
1O
2, O
2•−, OH
− overproduced by stress. This positive finding contributed to keeping the metabolic processes to improve plant performance [8,23,50–59]. Given the diversity of the key bioactive ingredients present in MEg-Sm as a focal biostimulator (
Table 5), it is a distinct strategy to treat maize plants as a foliar spray for the rapid growth of plants and efficient performance against Cd stress. A historical advance for controlling antioxidant genes for plant growth under stress has been reported by analyzing the transcriptional levels of antioxidant enzyme genes with the RT-qPCR technique [
8,
50]. Several enzyme genes are implicated in stressed plant development, and the molecular mechanisms of these antioxidant genes (e.g., SOD, CAT, APX, GR, and PrxQ) are linked with increased plant tolerance to stress [
8,
50]. In our study, along with the increase in nonenzymatic antioxidants, transcriptional levels of these genes increased under Cd stress and greatly increased with the application of MEg-Sm to maize plants (
Figure 5,
Table 6). These positive findings were reflected in the increased activity of SOD, CAT, APX, and GR enzymes (
Figure 4,
Table 6) to enable maize plants to effectively withstand Cd stress, increase their photosynthesis efficiency, and thus growth and dry matter accumulation (
Figure 1,
Table 6).
The role of silymarin, Sm (present in the MEg-Sm), as a secondary metabolite (Sm is a mixture of six flavonolignans (such as isosilybin A and B, silybin A and B, silydianin, and silychristin) and the flavonoid taxifolin), in improving plant performance under stress has not been achieved before. It has been reported that Sm can improve the productivity of plants since it accumulates in stressed plants to increase their defense systems [
74,
75]. This result is consistent with our results (
Figure 3,
Table 6). These reports [
74,
75] consider Sm as a powerful antioxidant, and thus its role in increasing plant resistance to stress is attributed to it as an antioxidant. Extensive studies are needed in this regard to exploring the precise mechanism of Sm for stress-tolerant plants.
The MEg-Sm results in this study are fully consistent with the characteristics of the biostimulator described by the European Biostimulant Industry Council [
76] and with the findings of other work examining different stresses [
3,
20,
21,
22,
23]. The growth promoters (bioactive compounds) present in MEg-Sm make it an effective biocatalyst and unique environmentally friendly strategy. MEg-Sm bioactive ingredients have functioned in this study to interplay with each other for successful plant growth under various stress conditions, including Cd stress. It has a high DPPH radical-scavenging activity (89.22%) because of its richness in various antioxidants, which possess high states of redox. This makes MEg-Sm possess pivotal mechanisms to prevent or suppress ROSs and lipid peroxidation as explained above. The complex interplaying of the bioactive ingredients of MEg-Sm occurred, in this study, to confer a robust defensive system against Cd-induced oxidative damage in favor of the performance of the maize plant.
The key ingredient biplot is a useful statistical method for assessing the interrelationship among the traits evaluated as well as the treatments examined [
77,
78]. In this study, the traits studied were divided into three groups. The traits exhibited a positive association with each other in the same group, whereas the traits with high positive values for PC1 displayed a negative association with those of negative PC1 values for PC1 [
79,
80]. Like the PC1, PC2 divided the treatments into two groups. The PC2 separated the applied treatments under Cd stress than those were performed under the absence of Cd on the other side of PC. Furthermore, the traits were identified in the first and third groups with treatments applied under Cd stress in the same sectors, indicating the importance of assessing these traits under Cd stress. These results are consistent with previous studies that showed the importance of physiological parameters as indicators under Cd stress [
81,
82,
83,
84].