New Generation of Hybrid Materials Based on Gelatin and Bioactive Glass Particles for Bone Tissue Regeneration
Abstract
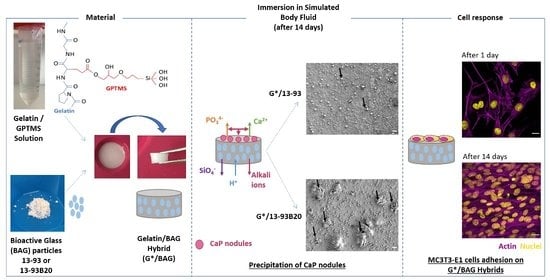
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation and Characterization
2.1.1. Bioactive Glass Processing
2.1.2. Hybrids Synthesis
2.2. Behavior of G*/BAG Hybrids
2.2.1. Physicochemical Properties of the Hybrids
Immersion in TRIS
Mineral Content in Hybrids
Mechanical Properties of the Hybrids
2.2.2. Hybrids Bioactivity
Immersion in Simulated Body Fluid (SBF)
Mineral Content in Hybrids
Hybrids Surface Analysis
2.2.3. Cell Analysis
Hybrids Preparation
Cell Culture
Cell Proliferation
Cell Morphology
Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Hybrids after Synthesis
3.2. Behavior of the Hybrids in Solution
3.2.1. Dissolution in TRIS
3.2.2. Dissolution in Simulated Body Fluid (SBF)
3.3. MC3T3-E1 Proliferation and Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31, S20–S22. [Google Scholar] [CrossRef] [PubMed]
- Myon, L.; Ferri, J.; Chai, F.; Blanchemain, N.; Raoul, G. Ingénierie du tissu osseux oro-maxillofacial par combinaison de biomatériaux, cellules souches, thérapie génique. Rev. De Stomatol. Et De Chir. Maxillo-Faciale 2011, 112, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, V.; Zivkovic, P.; Petrovic, D.; Stefanovic, V. Craniofacial Bone Tissue Engineering. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T. Apatite Formation on Surfaces of Ceramics, Metals and Polymers in Body Environment. Acta Mater. 1998, 46, 2519–2527. [Google Scholar] [CrossRef]
- Shimazaki, K.; Mooney, V. Comparative Study of Porous Hydroxyapatite and Tricalcium Phosphate as Bone Substitute. J. Orthop. Res. 1985, 3, 301–310. [Google Scholar] [CrossRef]
- Ohura, K.; Bohner, M.; Hardouin, P.; Lemaître, J.; Pasquier, G.; Flautre, B. Resorption of, and Bone Formation from, New β-Tricalcium Phosphate-Monocalcium Phosphate Cements: Anin Vivo Study. J. Biomed. Mater. Res. 1996, 30, 193–200. [Google Scholar] [CrossRef]
- Schwach, G.; Vert, M. In Vitro and in Vivo Degradation of Lactic Acid-Based Interference Screws Used in Cruciate Ligament Reconstruction. Int. J. Biol. Macromol. 1999, 25, 283–291. [Google Scholar] [CrossRef]
- Hasegawa, S.; Ishii, S.; Tamura, J.; Furukawa, T.; Neo, M.; Matsusue, Y.; Shikinami, Y.; Okuno, M.; Nakamura, T. A 5–7 Year in Vivo Study of High-Strength Hydroxyapatite/Poly(l-Lactide) Composite Rods for the Internal Fixation of Bone Fractures. Biomaterials 2006, 27, 1327–1332. [Google Scholar] [CrossRef]
- Verheyen, C.C.P.M.; de Wijn, J.R.; van Blitterswijk, C.A.; de Groot, K.; Rozing, P.M. Hydroxylapatite/Poly(L-Lactide) Composites: An Animal Study on Push-out Strengths and Interface Histology. J. Biomed. Mater. Res. 1993, 27, 433–444. [Google Scholar] [CrossRef]
- Ishikawa, K. Calcium Phosphate Cement. In Advances in Calcium Phosphate Biomaterials; Ben-Nissan, B., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2014; pp. 199–227. ISBN 978-3-642-53980-0. [Google Scholar]
- Hench, L.L. The Story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Day, R.M.; Boccaccini, A.R.; Shurey, S.; Roether, J.A.; Forbes, A.; Hench, L.L.; Gabe, S.M. Assessment of Polyglycolic Acid Mesh and Bioactive Glass for Soft-Tissue Engineering Scaffolds. Biomaterials 2004, 25, 5857–5866. [Google Scholar] [CrossRef]
- Houaoui, A.; Lyyra, I.; Agniel, R.; Pauthe, E.; Massera, J.; Boissière, M. Dissolution, Bioactivity and Osteogenic Properties of Composites Based on Polymer and Silicate or Borosilicate Bioactive Glass. Mater. Sci. Eng. C 2020, 107, 110340. [Google Scholar] [CrossRef]
- Mahony, O.; Tsigkou, O.; Ionescu, C.; Minelli, C.; Ling, L.; Hanly, R.; Smith, M.E.; Stevens, M.M.; Jones, J.R. Silica-Gelatin Hybrids with Tailorable Degradation and Mechanical Properties for Tissue Regeneration. Adv. Funct. Mater. 2010, 20, 3835–3845. [Google Scholar] [CrossRef]
- Novak, B.M. Hybrid Nanocomposite Materials-between Inorganic Glasses and Organic Polymers. Adv. Mater. 1993, 5, 422–433. [Google Scholar] [CrossRef]
- Nicole, L.; Boissière, C.; Grosso, D.; Quach, A.; Sanchez, C. Mesostructured Hybrid Organic–Inorganic Thin Films. J. Mater. Chem. 2005, 15, 3598. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Su, Y.-H.; Lai, J.-Y. In Situ Crosslinking of Chitosan and Formation of Chitosan–Silica Hybrid Membranes with Using γ-Glycidoxypropyltrimethoxysilane as a Crosslinking Agent. Polymer 2004, 45, 6831–6837. [Google Scholar] [CrossRef]
- Mahony, O.; Yue, S.; Turdean-Ionescu, C.; Hanna, J.V.; Smith, M.E.; Lee, P.D.; Jones, J.R. Silica–Gelatin Hybrids for Tissue Regeneration: Inter-Relationships between the Process Variables. J. Sol.-Gel Sci. Technol. 2014, 69, 288–298. [Google Scholar] [CrossRef] [Green Version]
- Vueva, Y.; Connell, L.S.; Chayanun, S.; Wang, D.; McPhail, D.S.; Romer, F.; Hanna, J.V.; Jones, J.R. Silica/Alginate Hybrid Biomaterials and Assessment of Their Covalent Coupling. Appl. Mater. Today 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Maeda, H.; Kasuga, T.; Hench, L.L. Preparation of Poly(l-Lactic Acid)-Polysiloxane-Calcium Carbonate Hybrid Membranes for Guided Bone Regeneration. Biomaterials 2006, 27, 1216–1222. [Google Scholar] [CrossRef]
- Vergnol, G.; Ginsac, N.; Rivory, P.; Meille, S.; Chenal, J.-M.; Balvay, S.; Chevalier, J.; Hartmann, D.J. In Vitro and in Vivo Evaluation of a Polylactic Acid-Bioactive Glass Composite for Bone Fixation Devices: Polylactic acid-bioactive glass composite for bone fixation devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 180–191. [Google Scholar] [CrossRef]
- Brink, M. The Influence of Alkali and Alkaline Earths on the Working Range for Bioactive Glasses. J. Biomed. Mater. Res. 1997, 36, 109–117. [Google Scholar] [CrossRef]
- Brown, R.F.; Rahaman, M.N.; Dwilewicz, A.B.; Huang, W.; Day, D.E.; Li, Y.; Bal, B.S. Effect of Borate Glass Composition on Its Conversion to Hydroxyapatite and on the Proliferation of MC3T3-E1 Cells. J. Biomed. Mater. Res. Part A 2009, 88A, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Ojansivu, M.; Mishra, A.; Vanhatupa, A.; Juntunen, M.; Larionova, A.; Massera, J.; Miettinen, S. The Effect of S53P4-Based Borosilicate Glasses and Glass Dissolution Products on the Osteogenic Commitment of Human Adipose Stem Cells. PLoS ONE 2018, 13, e0202740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maçon, A.L.B.; Kim, T.B.; Valliant, E.M.; Goetschius, K.; Brow, R.K.; Day, D.E.; Hoppe, A.; Boccaccini, A.R.; Kim, I.Y.; Ohtsuki, C.; et al. A Unified in Vitro Evaluation for Apatite-Forming Ability of Bioactive Glasses and Their Variants. J. Mater. Sci. Mater. Med. 2015, 26, 115. [Google Scholar] [CrossRef] [Green Version]
- Griffith, L.G.; Naughton, G. Tissue Engineering--Current Challenges and Expanding Opportunities. Science 2002, 295, 7. [Google Scholar] [CrossRef]
- Connell, L.S.; Gabrielli, L.; Mahony, O.; Russo, L.; Cipolla, L.; Jones, J.R. Functionalizing Natural Polymers with Alkoxysilane Coupling Agents: Reacting 3-Glycidoxypropyl Trimethoxysilane with Poly(γ-Glutamic Acid) and Gelatin. Polym. Chem. 2017, 8, 1095–1103. [Google Scholar] [CrossRef] [Green Version]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Wu, D.; Isaksson, P.; Ferguson, S.J.; Persson, C. Young’s Modulus of Trabecular Bone at the Tissue Level: A Review. Acta Biomater. 2018, 78, 1–12. [Google Scholar] [CrossRef]
- Bini, F.; Marinozzi, A.; Marinozzi, F.; Patanè, F. Microtensile Measurements of Single Trabeculae Stiffness in Human Femur. J. Biomech. 2002, 35, 1515–1519. [Google Scholar] [CrossRef]
- Hong, J.; Cha, H.; Park, Y.; Lee, S.; Khang, G.; Kim, Y. Elastic Moduli and Poisson’s Ratios of Microscopic Human Femoral Trabeculae. In IFMBE Proceedings, Proceedings of the11th Mediterranean Conference on Medical and Biomedical Engineering and Computing 2007, Ljubljana, Slovenia, 26–30 June 2007; Jarm, T., Kramar, P., Zupanic, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 16, pp. 274–277, ISBN 978-3-540-73043-9. [Google Scholar]
- Combes, C.; Cazalbou, S.; Rey, C. Apatite Biominerals. Minerals 2016, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Tan, C.; Abd Shukor, M.; Sopyan, I.; Teng, W. The Influence of Ca/P Ratio on the Properties of Hydroxyapatite Bioceramics. In Proceedings of the International Conference on Smart Materials and Nanotechnology in Engineering A, Harbin, China, 1–4 July 2007; Proceedings SPIE: Bellingham, WA, USA, 2007; Volume 6423. [Google Scholar] [CrossRef]
- Huang, W.; Day, D.E.; Kittiratanapiboon, K.; Rahaman, M.N. Kinetics and Mechanisms of the Conversion of Silicate (45S5), Borate, and Borosilicate Glasses to Hydroxyapatite in Dilute Phosphate Solutions. J. Mater. Sci. Mater. Med. 2006, 17, 583–596. [Google Scholar] [CrossRef]
- Stanislavov, A.S.; Sukhodub, L.F.; Sukhodub, L.B.; Kuznetsov, V.N.; Bychkov, K.L.; Kravchenko, M.I. Structural Features of Hydroxyapatite and Carbonated Apatite Formed under the Influence of Ultrasound and Microwave Radiation and Their Effect on the Bioactivity of the Nanomaterials. Ultrason. Sonochem. 2018, 42, 84–96. [Google Scholar] [CrossRef]
- Baddiel, C.B.; Berry, E.E. Spectra Structure Correlations in Hydroxy and Fluorapatite. Spectrochim. Acta 1966, 22, 1407–1416. [Google Scholar] [CrossRef]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. In Infrared Spectroscopy-Materials Science, Engineering and Technology; Theophanides, T., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0537-4. [Google Scholar]
- Maisani, M.; Pezzoli, D.; Chassande, O.; Mantovani, D. Cellularizing Hydrogel-Based Scaffolds to Repair Bone Tissue: How to Create a Physiologically Relevant Micro-Environment? J. Tissue Eng. 2017, 8, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Rahaman, M.N.; Bal, B.S.; Bonewald, L.F.; Kuroki, K.; Brown, R.F. Silicate, Borosilicate, and Borate Bioactive Glass Scaffolds with Controllable Degradation Rate for Bone Tissue Engineering Applications. II. In Vitro and in Vivo Biological Evaluation. J. Biomed. Mater. Res. Part A 2010, 95A, 172–179. [Google Scholar] [CrossRef]
- Eqtesadi, S.; Motealleh, A.; Pajares, A.; Miranda, P. Effect of Milling Media on Processing and Performance of 13-93 Bioactive Glass Scaffolds Fabricated by Robocasting. Ceram. Int. 2015, 41, 1379–1389. [Google Scholar] [CrossRef]










| Glass | mol% | ||||||
|---|---|---|---|---|---|---|---|
| Na2O | K2O | MgO | CaO | P2O5 | SiO2 | B2O3 | |
| 13-93 | 6.0 | 7.9 | 7.7 | 22.1 | 1.7 | 54.6 | - |
| 13-93B20 | 6.0 | 7.9 | 7.7 | 22.1 | 1.7 | 43.7 | 10.9 |
| Materials | Glass Loading in the Hybrids (wt%) | Young Modulus (MPa) | |
|---|---|---|---|
| Dry Samples | Wet Samples | ||
| G* alone | - | 2.1 ± 0.3 | 0.8 ± 0.2 |
| G*/13-93 | 34 ± 2 | 0.5 ± 0.3 | 0.5 ± 0.1 |
| G*/13-93B20 | 33 ± 1 | 0.7 ± 0.2 | 0.6 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houaoui, A.; Szczodra, A.; Lallukka, M.; El-Guermah, L.; Agniel, R.; Pauthe, E.; Massera, J.; Boissiere, M. New Generation of Hybrid Materials Based on Gelatin and Bioactive Glass Particles for Bone Tissue Regeneration. Biomolecules 2021, 11, 444. https://doi.org/10.3390/biom11030444
Houaoui A, Szczodra A, Lallukka M, El-Guermah L, Agniel R, Pauthe E, Massera J, Boissiere M. New Generation of Hybrid Materials Based on Gelatin and Bioactive Glass Particles for Bone Tissue Regeneration. Biomolecules. 2021; 11(3):444. https://doi.org/10.3390/biom11030444
Chicago/Turabian StyleHouaoui, Amel, Agata Szczodra, Mari Lallukka, Lamia El-Guermah, Remy Agniel, Emmanuel Pauthe, Jonathan Massera, and Michel Boissiere. 2021. "New Generation of Hybrid Materials Based on Gelatin and Bioactive Glass Particles for Bone Tissue Regeneration" Biomolecules 11, no. 3: 444. https://doi.org/10.3390/biom11030444