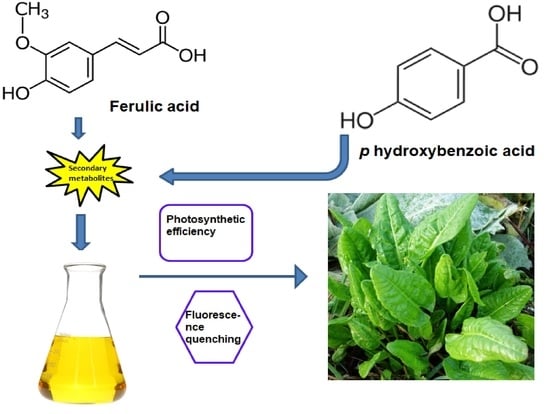
Secondary Metabolites, Ferulic Acid and p-Hydroxybenzoic Acid Induced Toxic Effects on Photosynthetic Process in Rumex acetosa L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phytotoxic Bioassays
2.2. Chlorophyll a Fluorescence Measurements
Calculations of the Electron Transport Rate
2.3. Statistical Analysis
3. Results
3.1. Effect of Allelochemicals on Photosynthetic Attributes and Photosystem II Photochemistry
3.2. Phytotoxicity of Allelochemicals in Light Harvesting Complex (LHC II)
3.3. Chlorophyll Fluorescence Quenching Analysis and Tolerance Potential of LHC
3.4. Impact of Phenolic Acids on Photon Energy Dissipation (1 − qP)/NPQ in R. acetosa
3.5. Photosynthetic Electron Transport (ETR) Responses under Allelochemical Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macías, F.A.; Mejías, F.J.; Molinillo, J.M. Recent advances in allelopathy for weed control: From knowledge to applications. Pest. Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Owen, D.K.; Duke, S.O. Rationale for a natural products approach to herbicide discovery. Pest. Manag. Sci. 2012, 68, 519–528. [Google Scholar] [CrossRef]
- Hussain, M.I.; Reigosa, M.J. Evaluation of photosynthetic performance and carbon isotope discrimination in perennial ryegrass (Lolium perenne L.) under allelochemicals stress. Ecotoxicology 2017, 26, 613–624. [Google Scholar] [CrossRef]
- Callaway, R.M.; Aschehoug, E.T. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science 2000, 290, 521–523. [Google Scholar] [CrossRef]
- Mwendwa, J.M.; Jeffrey, D.W.; Weston, L.A. The use of allelopathy and competitive crop cultivars for weed suppression in cereal crops. In Integrated Weed Management for Sustainable Agriculture; Burleigh Dodds Science Publishing: Sawston, UK, 2018; pp. 361–388. [Google Scholar]
- Hussain, M.I.; Reigosa, M.J. Higher peroxidase activity, leaf nutrient contents and carbon isotope composition changes in Arabidopsis thaliana are related to rutin stress. J. Plant Physiol. 2014, 171, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Durán, A.G.; Chinchilla, N.; Molinillo, J.M.; Macías, F.A. Structure-activity relationship studies on naphthoquinone analogs. The search for new herbicides based on natural products. Pest. Manag. Sci. 2019, 75, 2517–2529. [Google Scholar] [CrossRef] [PubMed]
- Einhellig, F.A. Allelopathy—A natural protection, allelochemicals. In Handbook of Natural Pesticides: Methods; CRC Press: Boca Raton, FL, USA, 2018; pp. 161–200. [Google Scholar]
- Dayan, F.E.; Duke, S.O. Natural compounds as next-generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.I.; González, L.; Chiapusio, G.; Reigosa, M.J. Benzoxazolin-2(3H)-one (BOA) induced changes in leaf water relations, photosynthesis and carbon isotope discrimination in Lactuca sativa. Plant Physiol. Biochem. 2011, 49, 825–834. [Google Scholar] [CrossRef]
- Doblinski, P.M.F.; Ferrarese, M.L.L.; Huber, D.A.; Scapim, C.A.; Braccini, A.L.; Ferrarese-Filho, O. Peroxidase and lipid peroxidation of soybean roots in response to p-coumaric and p-hydroxybenzoic acids. Braz. Arch. Biol. Technol. 2003, 46, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.I.; Reigosa, M.J.; Al-Dakheel, A.J. Biochemical, physiological and isotopic responses to natural product p-hydroxybenzoic acid in Cocksfoot (Dactylis glomerata L.). Plant Growth Regul. 2015, 75, 783–792. [Google Scholar] [CrossRef]
- Masum, S.M.; Hossain, M.A.; Akamine, H.; Sakagami, J.I.; Ishii, T.; Konno, T.; Nakamura, I. Comparison study of allelochemicals and bispyribac-sodium on the germination and growth response of Echinochloa crus-galli L. J. Plant. Growth Regul. 2019, 38, 501–512. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, W.; Wang, X.; Yang, L.; Han, S.; Chen, S.; Strasser, R.J.; Valverde, B.E.; Qiang, S. Comparative phytotoxicity of usnic acid, salicylic acid, cinnamic acid and benzoic acid on photosynthetic apparatus of Chlamydomonas reinhardtii. Plant Physiol. Biochem. 2018, 128, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Ferulic acid impairs rhizogenesis and root growth, and alters associated biochemical changes in mung bean (Vigna radiata) hypocotyls. J. Plant Interact. 2014, 9, 267–274. [Google Scholar] [CrossRef]
- Mitić, N.; Stanišić, M.; Savić, J.; Ćosić, T.; Stanisavljević, N.; Miljuš-Đukić, J.; Marin, M.; Radović, S.; Ninković, S. Physiological and cell ultrastructure disturbances in wheat seedlings generated by Chenopodium murale hairy root exudate. Protoplasma 2018, 255, 1683–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Liu, K.; Xie, Z.; Liu, M.; Chen, C. Effects of decomposing leaf litter of Leucaena leucocephala on photosynthetic traits of Cynodon dactylon and Medicago sativa. New For. 2018, 49, 667–679. [Google Scholar] [CrossRef]
- Hussain, M.I.; Reigosa, M.J. Allelochemical stress inhibits growth, leaf water relations, PSII photochemistry, non-photochemical fluorescence quenching and heat energy dissipation in three C3 perennial species. J. Expt. Bot. 2011, 62, 4533–4545. [Google Scholar] [CrossRef]
- Ladhari, A.; Gaaliche, B.; Zarrelli, A.; Ghannem, M.; Mimoun, M.B. Allelopathic potential and phenolic allelochemicals discrepancies in Ficus carica L. cultivars. S. Afr. J. Bot. 2020, 130, 30–44. [Google Scholar] [CrossRef]
- Inderjit; Mallik, A.U. Can Kalmia angustifolia interference to black spruce (Picea mariana) be explained by allelopathy? For. Ecol. Manag. 2002, 160, 75–84. [Google Scholar] [CrossRef]
- Hussain, M.I.; González, L.; Souto, C.; Reigosa, M.J. Ecophysiological responses of native plants to phytotoxic effect of Acacia melanoxylon R. Br. Agrofor. Syst. 2011, 83, 149–166. [Google Scholar] [CrossRef]
- Hussain, M.I.; Reigosa, M.J. A chlorophyll fluorescence analysis of photosynthetic efficiency, quantum yield and fractions of photon energy in PSII antennae of Lactuca sativa exposed to cinnamic acid. Plant Physiol. Biochem. 2011, 49, 1290–1298. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G. New fluorescence parameters for the determination of QA redox state and excitation energy. Photosyn. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Bilger, W.; Schreiber, U.; Bock, M. Determination of the quantum efficiency of photosystem II and of non-photochemical quenching of chlorophyll fluorescence in the field. Oecologia 1995, 102, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, X.; Che, Y.-h.; Wang, Y.; Li, M.-b.; Yang, R.-y.; Xu, N.; Sun, G.-y. A study on the effects of salinity and pH on PSII function in mulberry seedling leaves under saline–alkali mixed stress. Trees 2020, 34, 693–706. [Google Scholar]
- Hussain, M.I.; El-Keblawy, A. Surface canopy position determines the photosystem II photochemistry in invasive and native Prosopis congeners at Sharjah Desert, UAE. Forests 2020, 11, 740. [Google Scholar] [CrossRef]
- Liu, P.C.; Peacock, W.J.; Wang, L.; Furbank, R.; Larkum, A.; Dennis, E.S. Leaf growth in early development is key to biomass heterosis in Arabidopsis. J. Expt. Bot. 2020, 71, 2439–2450. [Google Scholar] [CrossRef]
- Demmig, B.; Björkman, O. Comparison of the effect of excessive light on chlorophyll fluorescence (77K) and photon yield of O2 evolution in leaves of higher plants. Planta 1987, 171, 171–184. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Bosa, K.; Kościelniak, J.; Żuk-Gołaszewska, K. Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ. Exp. Bot. 2011, 73, 64–72. [Google Scholar] [CrossRef]
- Hassannejad, S.; Lotfi, R.; Ghafarbi, S.P.; Oukarroum, A.; Abbasi, A.; Kalaji, H.M.; Rastogi, A. Early identification of herbicide modes of action by the use of chlorophyll fluorescence measurements. Plants 2020, 9, 529. [Google Scholar] [CrossRef]
- Einhellig, F.A. The physiology of allelochemical action: Clues and views. In Allelopathy: From Molecules to Ecosystems; Reigosa, M., Pedrol, N., Eds.; Science Publishers Inc.: Enfield, NH, USA, 2002; pp. 1–24. [Google Scholar]
- Pérez, F.J.; Ormeño-Núñez, J. Difference in hydroxamic acid content in roots and root exudates of wheat (Triticum aestivum L.) and rye (Secale cereale L.): Possible role in allelopathy. J. Chem. Ecol. 1991, 17, 1037–1043. [Google Scholar] [CrossRef]
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdowska, A. Allelochemicals as bioherbicides—Present and perspectives. In Herbicides-Current Research and Case Studies in Use; In Intech: London, UK, 2013. [Google Scholar]
- Al-Hamdi, B.; Inderjit; Olofsdotter, M.; Streibig, J.C. Laboratory bioassay for phytotoxicity: An example from wheat straw. Agron. J. 2001, 93, 43–48. [Google Scholar]
- Oliveira, K.S.; de Mello Prado, R.; de Farias Guedes, V.H. Leaf spraying of manganese with silicon addition is agronomically viable for corn and sorghum plants. J. Soil Sci. Plant Nutr. 2020, 20, 872–880. [Google Scholar] [CrossRef]
- Fricke, W.; Peters, W.S. The biophysics of leaf growth in salt-stressed barley. A study at the cell level. Plant Physiol. 2002, 129, 374–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, M.I.; Syed, Q.A.; Khattak, M.N.K.; Hafez, B.; Reigosa, M.J.; El-Keblawy, A. Natural product coumarins: Biological and pharmacological perspectives. Biologia 2019, 74, 863–888. [Google Scholar] [CrossRef]
- Hussain, M.I.; González, L.; Reigosa, M.J. Phytotoxic effect of allelochemicals and herbicides on photosynthesis, growth and carbon isotope discrimination in Lactuca sativa. Allelop. J. 2010, 26, 157–174. [Google Scholar]
- Hussain, M.I.; Semreen, M.H.; Shanableh, A.; Khattak, M.N.K.; Saadoun, I.; Ahmady, I.M.; Mousa, M.; Darwish, N.; Radeef, W.; Soliman, S.S. Phenolic composition and antimicrobial activity of different Emirati Date (Phoenix dactylifera L.) pits: A comparative study. Plants 2019, 8, 497. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Bie, Z. Cinnamic acid-inhibited ribulose-1,5-bisphosphate carboxylase activity is mediated through decreased spermine and changes in the ratio of polyamines in cowpea. J. Plant Physiol. 2010, 167, 47–53. [Google Scholar] [CrossRef]
- Ye, S.F.; Yu, J.Q.; Peng, Y.H.; Zheng, J.H.; Zou, L.Y. Incidence of Fusarium wilt in Cucumis sativus L. is promoted by cinnamic acid, an autotoxin in root exudates. Plant Soil 2004, 263, 143–150. [Google Scholar] [CrossRef]
- Nimbal, C.I.; Pedersen, J.F.; Yerkes, C.N.; Weston, L.A.; Weller, S.C. Phytotoxicity and distribution of sorgoleone in grain sorghum germplasm. J. Agric. Food Chem. 1996, 44, 1343–1347. [Google Scholar] [CrossRef]
- Nimbal, C.I.; Yerkes, C.N.; Weston, L.A.; Weller, S.C. Herbicidal activity and site of action of the natural product sorgoleone. Pestic. Biochem. Physiol. 1996, 54, 73–83. [Google Scholar] [CrossRef]
- Baker, N.R.; Nogués, S.; Allen, D.J. Photosynthesis and photoinhibition. In Seminar Series-Society for Experimental Biology; Cambridge University Press: Cambridge, UK, 1997; pp. 95–112. [Google Scholar]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant. Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [Green Version]
- Parizotto, A.V.; Marchiosi, R.; Bubna, G.A.; Bevilaqua, J.M.; Ferro, A.P.; Ferrarese, M.L.L.; Ferrarese-Filho, O. Benzoxazolin-2-(3H)-one reduces photosynthetic activity and chlorophyll fluorescence in soybean. Photosynthetica 2017, 55, 386–390. [Google Scholar] [CrossRef]
- Souza, R.P.; Machado, E.C.; Silva, J.A.B.; Lagôa, A.M.M.A.; Silveira, J.A.G. Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environ. Exp. Bot. 2004, 51, 45–56. [Google Scholar] [CrossRef]
- Sahu, G.K. Salicylic acid: Role in plant physiology and stress tolerance. In Molecular Stress Physiology of Plants; Springer: New Delhi, India, 2013; pp. 217–239. [Google Scholar]
- Chen, S.; Strasser, R.J.; Qiang, S. In vivo assessment of effect of phytotoxin tenuazonic acid on PSII reaction centers. Plant Physiol. Biochem. 2014, 84, 10–21. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence; Springer: Dordrecht, The Netherland, 2004; pp. 321–362. [Google Scholar]
- Inderjit; Duke, S.O. Ecophysiological aspect of allelopathy. Planta 2003, 217, 529–539. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, M.I.; Reigosa, M.J. Secondary Metabolites, Ferulic Acid and p-Hydroxybenzoic Acid Induced Toxic Effects on Photosynthetic Process in Rumex acetosa L. Biomolecules 2021, 11, 233. https://doi.org/10.3390/biom11020233
Hussain MI, Reigosa MJ. Secondary Metabolites, Ferulic Acid and p-Hydroxybenzoic Acid Induced Toxic Effects on Photosynthetic Process in Rumex acetosa L. Biomolecules. 2021; 11(2):233. https://doi.org/10.3390/biom11020233
Chicago/Turabian StyleHussain, M. Iftikhar, and Manuel J. Reigosa. 2021. "Secondary Metabolites, Ferulic Acid and p-Hydroxybenzoic Acid Induced Toxic Effects on Photosynthetic Process in Rumex acetosa L." Biomolecules 11, no. 2: 233. https://doi.org/10.3390/biom11020233