Extracellular Polymeric Substances Facilitate the Adsorption and Migration of Cu2+ and Cd2+ in Saturated Porous Media
Abstract
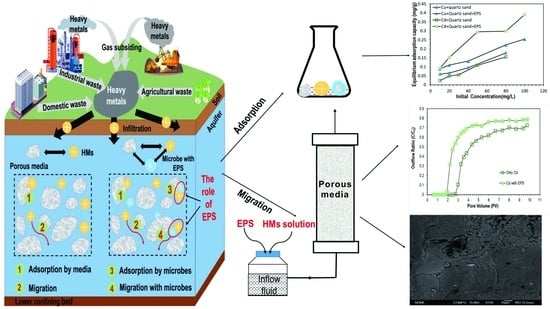
:1. Introduction
2. Materials and Methods
2.1. Preparation of Porous Materials
2.2. Extraction and Characterization of EPS
2.3. Procedure of Batch Experiments for Adsorption between EPS, Quartz Sand, and HMs
2.4. Apparatus and Setup for EPS/HMs Breakthroughs
2.5. Breakthrough of EPS/HMs in Porous Media
2.6. Determination of Cu2+ and Cd2+ Concentration
3. Results and Discussion
3.1. Analysis of EPS Composition
3.1.1. Effect of Dialysis on the Content of EPS Composition
3.1.2. Determination of the EPS Composition
3.2. Adsorption of EPS on Quartz Sand
3.3. Adsorption of Cu2+/Cd2+ by EPS
3.3.1. Adsorption of EPS with Single Metal Ion
3.3.2. Adsorption of Both Heavy Metals by EPS
3.4. Effect of EPS on Adsorption of Cu2+/Cd2+ on Quartz Sand
3.5. Breakthrough of EPS in Saturated Porous Media
3.6. Effect of EPS on the Cu2+/Cd2+ Migration through Saturated Porous Media
3.6.1. Effect of EPS on Migration of Individual Metal
3.6.2. Effect of EPS on Co-Transport of Cu2+ and Cd2+
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hu, B.; Shao, S.; Ni, H.; Fu, Z.Y.; Hu, L.S.; Zhou, Y.; Min, X.X.; She, S.F.; Chen, S.C.; Huang, M.X.; et al. Current status, spatial features, health risks, and potential driving factors of soil heavy metal pollution in China at province level. Environ. Pollut. 2020, 266, 114961. [Google Scholar] [CrossRef]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. Chembioeng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Cui, D.; Tan, C.; Deng, H.; Gu, X.; Pi, S.; Chen, T.; Zhou, L.; Li, A. Biosorption Mechanism of Aqueous Pb(2+), Cd(2+), and Ni(2+) Ions on Extracellular Polymeric Substances (EPS). Archaea 2020, 2020, 8891543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Wu, Q.; Wang, C.X.; Fu, X.; Wu, G. Impact of coal power generation on the characteristics and risk of heavy metal pollution in nearby soil. Ecosyst. Health Sustain. 2020, 6, 1787092. [Google Scholar] [CrossRef]
- Douay, F.; Pelfrene, A.; Planque, J.; Fourrier, H.; Richard, A.; Roussel, H.; Girondelot, B. Assessment of potential health risk for inhabitants living near a former lead smelter. Part 1: Metal concentrations in soils, agricultural crops, and homegrown vegetables. Environ. Monit. Assess. 2013, 185, 3665–3680. [Google Scholar] [CrossRef]
- Razack, M.; Furi, W.; Fanta, L.; Shiferaw, A. Water Resource Assessment of a Complex Volcanic System Under Semi-Arid Climate Using Numerical Modeling: The Borena Basin in Southern Ethiopia. Water 2020, 12, 276. [Google Scholar] [CrossRef] [Green Version]
- Khezzani, B.; Bouchemal, S. Variations in groundwater levels and quality due to agricultural over-exploitation in an arid environment: The phreatic aquifer of the Souf oasis (Algerian Sahara). Environ. Earth Sci. 2018, 77, 1–18. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Liang, X.; Jin, M.G.; Wan, L.; Yu, Q.C. Foundations of Hydrogeology, 6th ed.; Geological Publishing House: Beijing, China, 2011; p. 30. (In Chinese) [Google Scholar]
- Borsodi, A.K.; Micsinai, A.; Rusznyák, A.; Vladár, P.; Kovács, G.; Tóth, E.M.; Márialigeti, K. Diversity of alkaliphilic and alkalitolerant bacteria cultivated from decomposing reed rhizomes in a Hungarian soda lake. Microb. Ecol. 2005, 50, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Syngouna, V.I.; Chrysikopoulos, C.V. Cotransport of clay colloids and viruses in water saturated porous media. Colloids Surf. A Physicochem. Eng. Asp. 2013, 416, 56–65. [Google Scholar] [CrossRef]
- Flemming, H.-C. The perfect slime. Colloids Surf. B Biointerfaces 2011, 86, 251–259. [Google Scholar] [CrossRef]
- Decho, A.W.; Gutierrez, T. Microbial Extracellular Polymeric Substances (EPSs) in Ocean Systems. Front. Microbiol. 2017, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Neu, T.R.; Wingender, J. The Perfect Slime: Microbial Extracellular Polymeric Substances (EPS). Water Intell. Online 2016, 15, 9781780407425. [Google Scholar] [CrossRef]
- Wang, B.B.; Liu, X.T.; Chen, J.M.; Peng, D.C.; He, F. Composition and functional group characterization of extracellular polymeric substances (EPS) in activated sludge: The impacts of polymerization degree of proteinaceous substrates. Water Res. 2018, 129, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.P.; Yu, H.Q.; Li, X.Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Wang, H.; Qing, C.; Tan, T.; Shi, B.; Zhang, G.L.; Jiang, Z.; Wang, Y.H.; Hasan, S.Z. Arsenic mobilization affected by extracellular polymeric substances (EPS) of the dissimilatory iron reducing bacteria isolated from high arsenic groundwater. Sci. Total Environ. 2020, 735, 139501. [Google Scholar] [CrossRef]
- Xia, L.; Zheng, X.L.; Shao, H.B.; Xin, J.; Sun, Z.Y.; Wang, L.Y. Effects of bacterial cells and two types of extracellular polymers on bioclogging of sand columns. J. Hydrol. 2016, 535, 293–300. [Google Scholar] [CrossRef]
- Sun, X.F.; Wang, S.G.; Zhang, X.M.; Chen, J.P.; Li, X.M.; Gao, B.Y.; Ma, Y. Spectroscopic study of Zn2+ and Co2+ binding to extracellular polymeric substances (EPS) from aerobic granules. J. Colloid Interface Sci. 2009, 335, 11–17. [Google Scholar] [CrossRef]
- Wei, X.; Fang, L.C.; Cai, P.; Huang, Q.Y.; Chen, H.; Liang, W.; Rong, X.M. Influence of extracellular polymeric substances (EPS) on Cd adsorption by bacteria. Environ. Pollut. 2011, 159, 1369–1374. [Google Scholar] [CrossRef]
- Kantar, C.; Demiray, H.; Dogan, N.M. Role of microbial exopolymeric substances (EPS) on chromium sorption and transport in heterogeneous subsurface soils: II. Binding of Cr(III) in EPS/soil system. Chemosphere 2011, 82, 1496–1505. [Google Scholar] [CrossRef]
- Fang, L.; Wei, X.; Cai, P.; Huang, Q.Y.; Chen, H.; Liang, W.; Rong, X.M. Role of extracellular polymeric substances in Cu(II) adsorption on Bacillus subtilis and Pseudomonas putida. Bioresour. Technol. 2011, 102, 1137–1141. [Google Scholar] [CrossRef]
- Sheng, G.-P.; Xu, J.; Li, W.H.; Yu, H.Q. Quantification of the interactions between Ca2+, Hg2+ and extracellular polymeric substances (EPS) of sludge. Chemosphere 2013, 93, 1436–1441. [Google Scholar] [CrossRef]
- Wei, D.; Li, M.T.; Wang, X.D.; Han, F.; Li, L.S.; Guo, J.; Ai, L.J.; Fang, L.L.; Liu, L.; Du, B.; et al. Extracellular polymeric substances for Zn (II) binding during its sorption process onto aerobic granular sludge. J. Hazard. Mater. 2016, 301, 407–415. [Google Scholar] [CrossRef]
- Chen, J.-H.; Lion, L.W.; Ghiorse, W.C.; Shuler, M.L. Mobilization of adsorbed cadmium and lead in aquifer material by bacterial extracellular polymers. Water Res. 1995, 29, 421–430. [Google Scholar] [CrossRef]
- Xia, L.; Tan, J.Q.; Wu, P.Y.; He, Q.N.; Song, S.X.; Li, Y.T. Biopolymers extracted from Klebsiella sp. and Bacillus sp. in wastewater sludge as superb adsorbents for aqueous Hg(II) removal from water. Chem. Phys. Lett. 2020, 754, 137689. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Li, M.M.; Chen, T.H.; Zhou, Y.F.; Yue, Z.B. Competitive adsorption of heavy metal by extracellular polymeric substances (EPS) extracted from sulfate reducing bacteria. Bioresour. Technol. 2014, 163, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.R.; Hu, Y.Y.; Xiong, F. Sorption of Cu(II) and Cd(II) by extracellular polymeric substances (EPS) from Aspergillus fumigatus. Int. Biodeterior. Biodegrad. 2011, 65, 1012–1018. [Google Scholar] [CrossRef]
- Wei, W.; Wang, Q.L.; Li, A.; Yang, J.X.; Ma, F.; Pi, S.S.; Wu, D. Biosorption of Pb (II) from aqueous solution by extracellular polymeric substances extracted from Klebsiella sp J1: Adsorption behavior and mechanism assessment. Sci. Rep. 2016, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Perujo, N.; Romani, A.M.; Sanchezvila, X. A bilayer coarse-fine infiltration system minimizes bioclogging: The relevance of depth-dynamics. Sci. Total Environ. 2019, 669, 559–569. [Google Scholar] [CrossRef]
- Jia, C.; Li, P.J.; Li, X.J.; Tai, P.D.; Liu, W.; Gong, Z.Q. Degradation of pyrene in soils by extracellular polymeric substances (EPS) extracted from liquid cultures. Process Biochem. 2011, 46, 1627–1631. [Google Scholar] [CrossRef]
- Zhang, X.; Bishop, P.L.; Kinkle, B.K. Comparison of extraction methods for quantifying extracellular polymers in biofilms. Water Sci. Technol. 1999, 39, 211–218. [Google Scholar] [CrossRef]
- Cao, F.; Bourven, I.; Hullebusch, E.D.; Pechaud, Y.; Lens, P.N.L.; Guibaud, G. Hydrophobic molecular features of EPS extracted from anaerobic granular sludge treating wastewater from a paper recycling plant. Process Biochem. 2017, 58, 266–275. [Google Scholar] [CrossRef]
- Zhao, L.T.; She, Z.L.; Jin, C.J.; Yang, S.Y.; Guo, L.; Zhao, Y.G.; Gao, M.C. Characteristics of extracellular polymeric substances from sludge and biofilm in a simultaneous nitrification and denitrification system under high salinity stress. Bioprocess Biosyst. Eng. 2016, 39, 1375–1389. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Ren, H.Q.; Ding, L.L. Comparison of extracellular polymeric substances (EPS) extraction from two different activated sludges. Water Sci. Technol. 2012, 66, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Li, W.W.; Yu, H.Q.; Harada, H. A novel integrated approach to quantitatively evaluate the efficiency of extracellular polymeric substances (EPS) extraction process. Appl. Microbiol. Biotechnol. 2012, 96, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Xu, T.; Nie, Q.K.; Li, P.P. Temperature-driven migration of heavy metal Pb2+ along with moisture movement in unsaturated soils. Int. J. Heat Mass Transf. 2020, 153, 119573. [Google Scholar] [CrossRef]
- Peng, C.S.; Almeira, J.O.; Abou-Shady, A. Enhancement of ion migration in porous media by the use of varying electric fields. Sep. Purif. Technol. 2013, 118, 591–597. [Google Scholar] [CrossRef]
- Li, X.H.; Xu, H.X.; Gao, B.; Yang, Z.D.; Sun, Y.Y.; Shi, X.Q.; Wu, J.C. Cotransport of Herbaspirillum chlorophenolicum FA1 and heavy metals in saturated porous media: Effect of ion type and concentration. Environ. Pollut. 2019, 254, 112940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Xia, S.Q.; Wang, X.J.; Yang, A.; Xu, B.; Chen, L.; Zhu, Z.L.; Zhao, J.F.; Jaffrezic-Renault, N.; Leonard, D. A novel biosorbent for dye removal: Extracellular polymeric substance (EPS) of Proteus mirabilis TJ-1. J. Hazard. Mater. 2009, 163, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Adav, S.S.; Lee, D. Extraction of extracellular polymeric substances from aerobic granule with compact interior structure. J. Hazard. Mater. 2008, 154, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- D’Abzac, P.; Bordas, F.; Hullebusch, V.E.; Lens, P.N.L.; Guibaud, G. Extraction of extracellular polymeric substances (EPS) from anaerobic granular sludges: Comparison of chemical and physical extraction protocols. Appl. Microbiol. Biotechnol. 2010, 85, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.Q.; Allen, D.G.; Droppo, I.G.; Leppard, G.G.; Liss, S.N. Surface properties of sludge and their role in bioflocculation and settleability. Water Res. 2001, 35, 339–350. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Zhou, Y.; Zhang, J.; Xia, S.Q. Copper (II) adsorption by the extracellular polymeric substance extracted from waste activated sludge after short-time aerobic digestion. Environ. Sci. Pollut. Res. 2014, 21, 2132–2140. [Google Scholar] [CrossRef] [PubMed]
- Chouyyok, W.; Shin, Y.; Davidson, J.; Samuels, W.D.; LaFemina, N.H.; Rutledge, R.D.; Fryxell, G.E.; Sangvanich, T.; Yantasee, W. Selective Removal of Copper(II) from Natural Waters by Nanoporous Sorhents Functionalized with Chelating Diamines. Environ. Sci. Technol. 2010, 44, 6390–6395. [Google Scholar] [CrossRef] [Green Version]
- Kumar, Y.P.; King, P.; Prasad, V.S.R.K. Equilibrium and kinetic studies for the biosorption system of copper(II) ion from aqueous solution using Tectona grandis L.f. leaves powder. J. Hazard. Mater. 2006, 137, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.-P.; Xu, J.; Luo, H.W.; Li, W.W.; Li, W.H.; Yu, H.Q.; Xie, Z.; Wei, S.Q.; Hu, F.C. Thermodynamic analysis on the binding of heavy metals onto extracellular polymeric substances (EPS) of activated sludge. Water Res. 2013, 47, 607–614. [Google Scholar] [CrossRef]
- Fang, L.C.; Huang, Q.Y.; Wei, X.; Liang, W.; Rong, X.M.; Chen, W.L.; Cai, P. Microcalorimetric and potentiometric titration studies on the adsorption of copper by extracellular polymeric substances (EPS), minerals and their composites. Bioresour. Technol. 2010, 101, 5774–5779. [Google Scholar] [CrossRef]
- Comte, S.; Guibaud, G.; Baudu, M. Biosorption properties of extracellular polymeric substances (EPS) towards Cd, Cu and Pb for different pH values. J. Hazard. Mater. 2008, 151, 185–193. [Google Scholar] [CrossRef]
- Guine, V.; Martins, J.; Gaudet, J.P. Facilitated transport of heavy metals by bacterial colloids in sand columns. J. Phys. IV 2003, 107, 593–596. [Google Scholar] [CrossRef]
- Pang, L.; Close, M.E.; Noonan, M.J.; Flintoft, M.J.; van den Brink, P. A laboratory study of bacteria-facilitated cadmium transport in alluvial gravel aquifer media. J. Environ. Qual. 2005, 34, 237–247. [Google Scholar] [CrossRef] [Green Version]






| The Sources of Microorganisms | Microorganisms Types | The Kind of Metal | Adsorption Capacity or Adsorption Efficiency | Reference |
|---|---|---|---|---|
| Wastewater sludge systems | Klebsiella sp., Bacillus sp. | Hg(II) | 2597.62 mg/g (Klebsiella sp.), 2617.23 mg/g (Bacillus sp.) | [25] |
| Aqueous environment | Agrobacterium tumefaciens F2 | Pb2+, Cd2+, and Ni2+ | 94.67% (Pb2+), 94.41% (Cd2+), 77.95% (Ni2+) | [3] |
| Wastewater treat plant | D. desulfuricans (GenBank/HQ022824.1) | Cu2+, Zn2+ | 899.1 mg/g EPS for Cu2+, 932.1 mg/g EPS for Zn2+ | [26] |
| - | Aspergillus fumigatus | Cu(II), Cd(II) | 40 mg/g EPS for Cu(II), 85.5 mg/g EPS for Cd(II) | [27] |
| Activated sludge in municipal wastewater treatment plants | Klebsiella sp. J1 | Pb(II) | 99.5 mg/g | [28] |
| No. | Concentration of Cu (mg/L) | Concentration of Cd (mg/L) | Contact Time (min) |
|---|---|---|---|
| 1 | 5 | 0 | 5, 15, 30, 50, 70, 100, 120, 240, 360, 720 |
| 2 | 5, 10, 15, 25, 40 | 0 | 720 |
| 3 | 0 | 7 | 5, 15, 30, 50, 70, 100, 240, 720 |
| 4 | 0 | 10, 20, 30, 50, 80 | 720 |
| 5 | 0 | 5 | 720 |
| 6 | 0 | 7 | 720 |
| 7 | 5 | 7 | 720 |
| No. | Hyperpure Water | EPS (PV) (50 mg/L) | Cu2+ (PV) (100 mg/L) | Cd2+ (PV) (50 mg/L) |
|---|---|---|---|---|
| 1 | 4 | 13 | - | - |
| 2 | 4 | 13 | 10 | - |
| 2 * | 17 | - | 10 | - |
| 3 | 4 | 13 | - | 10 |
| 3 * | 17 | - | - | 10 |
| 4 | 4 | 13 | 5 | 5 |
| 4 * | 4 | - | 5 | 5 |
| Isothermal Adsorption Model | Pseudo-Second-Order Kinetic Model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Freundlich Constants | Linear Model Constants | Temkin Constants | |||||||||
| K | n | R2 | K | R2 | K | R2 | Qe,exp (mg/g) | K (g/(mg h)) | R2 | Qe,cal (mg/g) | |
| Cu2+ | 14.02 | 1.409 | 0.904 | 3.908 | 0.883 | 44.704 | 0.963 | 20.79 | 2.208 | 0.993 | 13.46 |
| Cd2+ | 0.372 | 0.697 | 0.974 | 2.327 | 0.956 | 66.496 | 0.894 | 15.93 | −0.568 | 0.997 | 14.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Li, Z.; Yang, Y.; Purchase, D.; Lu, Y.; Dai, Z. Extracellular Polymeric Substances Facilitate the Adsorption and Migration of Cu2+ and Cd2+ in Saturated Porous Media. Biomolecules 2021, 11, 1715. https://doi.org/10.3390/biom11111715
Wu Y, Li Z, Yang Y, Purchase D, Lu Y, Dai Z. Extracellular Polymeric Substances Facilitate the Adsorption and Migration of Cu2+ and Cd2+ in Saturated Porous Media. Biomolecules. 2021; 11(11):1715. https://doi.org/10.3390/biom11111715
Chicago/Turabian StyleWu, Yuhui, Zhengyu Li, Yuesuo Yang, Diane Purchase, Ying Lu, and Zhenxue Dai. 2021. "Extracellular Polymeric Substances Facilitate the Adsorption and Migration of Cu2+ and Cd2+ in Saturated Porous Media" Biomolecules 11, no. 11: 1715. https://doi.org/10.3390/biom11111715