Endothelial Dysfunction and Extra-Articular Neurological Manifestations in Rheumatoid Arthritis
Abstract
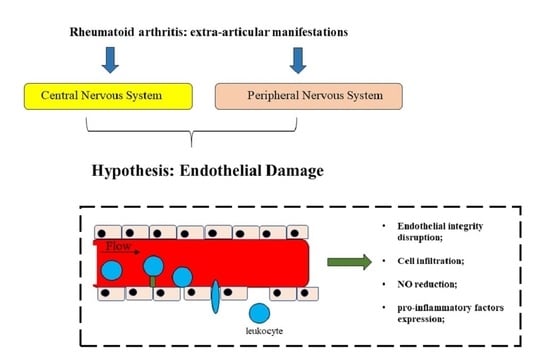
:1. Introduction
2. Extra-Articular Manifestations in Rheumatoid Arthritis
2.1. Multiorgan RA-Associated Disease
2.2. Neurological Disorders Associated to RA
2.3. Cognitive Impairment in Rheumatoid Arthritis
3. The Pathophysiology of Endothelial Dysfunction in RA
3.1. RA-Associted Inflammation and Endothelial Dysfunction
3.2. Endoplasmic Reticulum Stress in Endothelial Cells of Patients with RA
3.3. The Role of Dysfunctional Endothelium in the Release of Neurotrophic Factors in RA
3.4. Disruption of Brain Nerve Barrier (BNB) and its Role on RA-Associated Neurological Manifestation
- a reduction of NO;
- a greater expression of pro-inflammatory factors; and
- a modification of the permeability of the endothelium [100].
- higher incidence of RA in women, during the fertile sexual period, compared to men (ratio 3:1);
- menopausal remissions in women with RA [116];
- improvements in the course of the disease in approximately 75% of women during pregnancy. It is important to underline the shift that 17-βestradiol undergoes during pregnancy. In this circumstance, the function of the sex hormone is mainly anti-inflammatory with marked inhibition of pro-inflammatory cytokines, such as tumor necrosis factor, IL-1β, IL-6, and natural killer cells [117].
4. The Impact of Lifestyle Changes in RA
Rheumatoid Arthritis and Nutrition: Involvement of Endothelium
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roberts, M.H.; Erdei, E. Comparative United States autoimmune disease rates for 2010–2016 by sex, geographic region, and race. Autoimmun. Rev. 2020, 19, 102423. [Google Scholar] [CrossRef]
- Croia, C.; Bursi, R.; Sutera, D.; Petrelli, F.; Alunno, A.; Puxeddu, I. One year in review 2019: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2019, 37, 347–357. [Google Scholar]
- Bemis, E.A.; Norris, J.M.; Seifert, J.; Frazer-Abel, A.; Okamoto, Y.; Feser, M.L.; Demoruelle, M.K.; Deane, K.D.; Banda, N.K.; Holers, V.M. Complement and its environmental determinants in the progression of human rheumatoid arthritis. Mol. Immunol. 2019, 112, 256–265. [Google Scholar] [CrossRef]
- Mori, H.; Sawada, T.; Nishiyama, S.; Shimada, K.; Tahara, K.; Hayashi, H.; Kato, E.; Tago, M.; Matsui, T.; Tohma, S. Influence of seasonal changes on disease activity and distribution of affected joints in rheumatoid arthritis. BMC Musculoskelet. Disord. 2019, 20, 30. [Google Scholar] [CrossRef]
- Horita, M.; Nishida, K.; Hashizume, K.; Nasu, Y.; Saiga, K. Outcomes of Resection and Joint Preserving Arthroplasty for Forefoot Deformities for Rheumatoid Arthritis. Foot Ankle Int. 2018, 39, 292–299. [Google Scholar] [CrossRef]
- Entezami, P.; Fox, D.A.; Clapham, P.J.; Chung, K.C. Historical perspective on the etiology of rheumatoid arthritis. Hand Clin. 2011, 27, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kobak, S.; Bes, C. An autumn tale: Geriatric rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2018, 10, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Marcucci, E.; Bartoloni, E.; Alunno, A.; Leone, M.C.; Cafaro, G.; Luccioli, F.; Valentini, V.; Valentini, E.; La Paglia, G.M.C.; Bonifacio, A.F.; et al. Extra-articular rheumatoid arthritis. Reumatismo 2018, 70, 212–224. [Google Scholar] [CrossRef] [Green Version]
- Schrepf, A.; Kaplan, C.M.; Ichesco, E.; Larkin, T.; Harte, S.E.; Harris, R.E.; Murray, A.D.; Waiter, G.D.; Clauw, D.J.; Basu, N. A multi-modal mri study of the central response to infammation in rheumatoid arthritis. Nat. Commun. 2018, 9, 2243. [Google Scholar] [CrossRef]
- Fiest, K.M.; Hitchon, C.A.; Bernstein, C.N.; Peschken, C.A.; Walker, J.R.; Graff, L.A.; Zarychanski, R.; Abou-Setta, A.; Patten, S.B.; Sareen, J.; et al. Systematic review and meta-analysis of interventions for depression and anxiety in persons with rheumatoid arthritis. J. Clin. Rheumatol. 2017, 23, 425–434. [Google Scholar] [CrossRef]
- Bernatova, I.; Andriantsitohaina, R.; Arribas, S.M.; Matchkov, V.V. Endothelium in diseased states. Biomed. Res. Int. 2014, 2014, 810436. [Google Scholar] [CrossRef] [Green Version]
- Segarra, M.; Aburto, M.R.; Hefendehl, J.; Acker-Palmer, A. Neurovascular Interactions in the Nervous System. Annu. Rev. Cell Dev. Biol. 2019, 35, 615–635. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Kishore, S.; Maher, L.; Vikas, M. Rheumatoid vasculitis: A diminishing yet devastating menace. Curr. Rheumatol. Rep. 2017, 19, 39. [Google Scholar] [CrossRef]
- Choy, E.; Ganeshalingam, K.; Semb, A.G.; Szekanecz, Z.; Nurmohamed, M. Cardiovascular risk in rheumatoid arthritis: Recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology (Oxford) 2014, 53, 2143–2154. [Google Scholar] [CrossRef] [Green Version]
- Lauper, K.; Gabay, C. Cardiovascular risk in patients with rheumatoid arthritis. Semin. Immunopathol. 2017, 39, 447–459. [Google Scholar] [CrossRef]
- Charles-Schoeman, C.; Lee, Y.Y.; Grijalva, V.; Amjadi, S.; FitzGerald, J.; Ranganath, V.K.; Taylor, M.; McMahon, M.; Paulus, H.E.; Reddy, S.T. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann. Rheum. Dis. 2014, 71, 1157–1162. [Google Scholar] [CrossRef]
- Kerekes, G.; Nurmohamed, M.T.; González-Gay, M.A.; Seres, I.; Paragh, G.; Kardos, Z.; Baráth, Z.; Tamási, L.; Soltész, P.; Szekanecz, Z. Rheumatoid arthritis and metabolic syndrome. Nat. Rev. Rheumatol. 2014, 10, 691–696. [Google Scholar] [CrossRef]
- Bartoloni, E.; Alunno, A.; Gerli, R. Hypertension as a cardiovascular risk factor in autoimmune rheumatic diseases. Nat. Rev. Cardiol. 2018, 15, 33–44. [Google Scholar] [CrossRef]
- Corrao, S.; Messina, S.; Pistone, G.; Calvo, L.; Scaglione, R.; Licata, G. Heart involvement in rheumatoid arthritis: Systematic review and meta-analysis. Int. J. Cardiol. 2013, 167, 2031–2038. [Google Scholar] [CrossRef] [Green Version]
- Lora, V.; Cerroni, L.; Cota, C. Skin manifestations of rheumatoid arthritis. G. Ital. Dermatol. Venereol. 2018, 153, 243–255. [Google Scholar]
- Ziemer, M.; Müller, A.K.; Hein, G.; Oelzner, P.; Elsner, P. Incidence and classification of cutaneous manifestations in rheumatoid arthritis. J. Dtsch. Dermatol. Ges. 2016, 14, 1237–1246. [Google Scholar] [CrossRef]
- Nyhäll-Wåhlin, B.M.; Turesson, C.; Jacobsson, L.T.H.; Nilsson, J.A.; Forslind, K.; Albertsson, K.; Rönnelid, J.; Petersson, I.F. The presence of rheumatoid nodules at early rheumatoid arthritis diagnosis is a sign of extra-articular disease and predicts radiographic progression of joint destruction over 5 years. Scand. J. Rheumatol. 2011, 40, 81–87. [Google Scholar] [CrossRef]
- Kaushik, P.; Solomon, D.H.; Greenberg, J.D.; Anderson, J.T.; Reed, G.; Pala, O.; Sumbul-Yuksel, B.; Kadam, P.; Kremer, J.M. Subcutaneous nodules are associated with cardiovascular events in patients with rheumatoid arthritis: Results from a large US registry. Clin. Rheumatol. 2015, 34, 1697–1704. [Google Scholar] [CrossRef]
- O’Dwyer, D.N.; Armstrong, M.E.; Cooke, G.; Dodd, J.D.; Veale, D.J.; Donnelly, S.C. Rheumatoid arthritis (RA) associated interstitial lung disease (ILD). Eur. J. Int. Med. 2013, 24, 597–603. [Google Scholar] [CrossRef]
- Sihvonen, S.; Korpela, M.; Laippala, P.; Mustonen, J.; Pasternack, A. Death rates and causes of death in patients with rheumatoid arthritis: A population-based study. Scand. J. Rheumatol. 2004, 33, 221–227. [Google Scholar] [CrossRef]
- Clive, K.; Kundan, I.; Iman-Gutierrez, L. Lung involvement in inflammatory rheumatic diseases. Best Pract. Res. Clin. Rheumatol. 2016, 30, 870–888. [Google Scholar]
- Craig, E.; Cappelli, L.C. Gastrointestinal and Hepatic Disease in Rheumatoid Arthritis. Rheum Dis. Clin. N. Am. 2018, 44, 89–111. [Google Scholar] [CrossRef]
- Kröner, P.T.; Tolaymat, O.A.; Bowman, A.W.; Abril, A.; Lacy, B.E. Gastrointestinal Manifestations of Rheumatological Diseases. Am. J. Gastroenterol. 2019, 114, 1441–1454. [Google Scholar] [CrossRef]
- Radovanović-Dinić, B.; Tešić-Rajković, S.; Zivkovi, V.; Grgov, S. Clinical connection between rheumatoid arthritis and liver damage. Rheumatol. Int. 2018, 38, 715–724. [Google Scholar] [CrossRef]
- Adami, G.; Saag, K.G. Osteoporosis Pathophysiology, Epidemiology, and Screening in Rheumatoid Arthritis. Curr. Rheumatol. Rep. 2019, 21, 34. [Google Scholar] [CrossRef]
- Krumbholz, G.; Junker, S.; Meier, F.M.P.; Rickert, M.; Steinmeyer, J.; Rehart, S.; Lange, U.; Frommer, K.W.; Schett, G.; Müller-Ladner, U.; et al. Response of human rheumatoid arthritis osteoblasts and osteoclasts to adiponectin. Clin. Exp. Rheumatol. 2017, 35, 406–414. [Google Scholar]
- Masamoto, K.; Otsuki, B.; Fujibayashi, S.; Shima, K.; Ito, H.; Furu, M.; Hashimoto, M.; Tanaka, M.; Lyman, S.; Yoshitomi, H.; et al. Factors influencing spinal sagittal balance, bone mineral density, and Oswestry Disability Index outcome measures in patients with rheumatoid arthritis. Eur. Spine J. 2018, 27, 406–415. [Google Scholar] [CrossRef]
- Lee, Y.H.; Bae, S.C. Vitamin D level in rheumatoid arthritis and its correlation with the disease activity: A meta-analysis. Clin. Exp. Rheumatol. 2016, 34, 827–833. [Google Scholar]
- Giles, J.T. Extra-articular Manifestations and Comorbidity in Rheumatoid Arthritis: Potential Impact of Pre-Rheumatoid Arthritis Prevention. Clin. Ther. 2019, 41, 1246–1255. [Google Scholar] [CrossRef]
- Charen, D.A.; Markowitz, J.S.; Cheung, Z.B.; Matijakovich, D.J.; Chan, J.J.; Vulcano, E. Overview of Metatarsalgia. Orthopedics 2019, 42, e138–e143. [Google Scholar]
- Kothe, R. Rheumatoid instability in the cervical spine: Diagnostic and therapeutic strategies. Orthopade 2018, 47, 489–495. [Google Scholar] [CrossRef]
- McKenna, M.C.; Vaughan, D.; Bermingham, N.; Cronin, S. Rheumatoid arthritis presenting as rheumatoid meningitis. BMJ Case Rep. 2019, 12, bcr-2018-226649. [Google Scholar] [CrossRef]
- Bang, S.; Kim, Y.; Jang, K.; Paik, S.S.; Shin, S.J. Clinicopathologic features of rheumatoid nodules: A retrospective analysis. Clin. Rheumatol. 2019, 38, 3041–3048. [Google Scholar] [CrossRef]
- Carotti, M.; Salaffi, F.; di Carlo, M.; Sessa, F.; Giovagnoni, A. Magnetic resonance imaging of the craniovertebral junction in early rheumatoid arthritis. Skelet. Radiol. 2019, 48, 553–561. [Google Scholar] [CrossRef]
- Janssen, I.; Nouri, A.; Tessitore, E.; Meyer, B. Cervical Myelopathy in Patients Suffering from Rheumatoid Arthritis-A Case Series of 9 Patients and A Review of the Literature. J. Clin. Med. 2020, 9, 811. [Google Scholar] [CrossRef] [Green Version]
- Ulutatar, F.; Unal-Ulutatar, C.; Duruoz, M.T. Cervical proprioceptive impairment in patients with rheumatoid arthritis. Rheumatol. Int. 2019, 39, 2043–2051. [Google Scholar] [CrossRef]
- Geraldo-Flores, N.A.; Merlos-López, R.J.; Rodríguez-Wong, J.A.; Ramírez-Hernández, S.; Espino-Lizarraga, M.J.; Pérez-Atanasio, J.M. The severity of rheumatoid arthritis as a timely predictor of instability in the asymptomatic cervical spine. Acta Ortop. Mex. 2018, 32, 342–346. [Google Scholar]
- Meyer, C.; Bredow, J.; Heising, E.; Eysel, P.; Müller, L.P.; Stein, G. Rheumatoid Arthritis Affecting the Upper Cervical Spine: Biomechanical Assessment of the Stabilizing Ligaments. Biomed. Res. Int. 2017, 2017, 6131703. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.; Masiak, A.; Zdrojewski, Z. Rheumatoid arthritis with pachymeningitis—A case presentation and review of the literature. Reumatologia 2020, 58, 116–122. [Google Scholar] [CrossRef]
- Oono, M.; Fujita, Y.; Uchida, N.; Kawai, U.; Fujita-Nakata, M.; Nakanishi, M.; Sanada, M.; Nagayama, S.; Matsui, M. Rheumatoid meningitis developed in patient with stable rheumatoid arthritis and myasthenia gravis—detailed analysis of intracranial inflammation using flow cytometry. J. Neuroinflamm. 2018, 15, 151. [Google Scholar] [CrossRef] [Green Version]
- Abussuud, Z.A.; Geneta, V.P. Rheumatoid Meningitis. World Neurosurg. 2020, 137, 98–101. [Google Scholar] [CrossRef]
- Nissen, M.S.; Nilsson, A.C.; Forsberg, J.; Milthers, J.; Wirenfeldt, M.; Bonde, C.; Byg, K.E.; Ellingsen, T.; Blaabjerg, M. Use of Cerebrospinal Fluid Biomarkers in Diagnosis and Monitoring of Rheumatoid Meningitis. Front. Neurol. 2019, 10, 666. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Cohen, J.M.; Wright, N.A.; Merola, J.F. Skin Signs of Rheumatoid Arthritis and its Therapy-Induced Cutaneous Side Effects. Am. J. Clin. Dermatol. 2016, 17, 147–162. [Google Scholar] [CrossRef]
- Abdullah, H.M.A.; Omar, M.; Jbeli, A.; Fanciullo, J. Meningeal rheumatoid nodules in a 55-year-old man presenting with chronic headaches and oculomotor nerve palsy: An uncommon extra-articular manifestation of rheumatoid arthritis. BMJ Case Rep. 2019, 12, e231474. [Google Scholar] [CrossRef]
- DeQuattro, K.; Imboden, J.B. Neurologic Manifestations of Rheumatoid Arthritis. Rheum. Dis. Clin. N. Am. 2017, 43, 561–571. [Google Scholar] [CrossRef]
- Trăistaru, M.R.; Kamal, D.; Traşcă, D.M.; Foarfă, M.C.; Gruia, C.L.; Rogoveanu, O.C. Rheumatoid nodules and quality of life in rheumatoid arthritis females—complex assessment. Rom. J. Morphol. Embryol. 2016, 57, 215–225. [Google Scholar]
- Luís, M.; Freitas, J.; Costa, F.; Buttgereit, F.; Boers, M.; Jap, D.S.; Santiago, T. An updated review of glucocorticoid-related adverse events in patients with rheumatoid arthritis. Expert Opin. Drug Saf. 2019, 18, 581–590. [Google Scholar] [CrossRef]
- Bhamra, M.S.; Gondal, I.; Amarnani, A.; Betesh, S.; Zhyvotovska, A.; Scott, W.; Rodriguez-Alvarez, M.; Lazzaro, D.R.; McFarlane, I.M. Ocular Manifestations of Rheumatoid Arthritis: Implications of Recent Clinical Trials. Int. J. Clin. Res. Trials 2019, 4, 139. [Google Scholar] [CrossRef] [Green Version]
- Peterson, S.; Piercy, J.; Blackburn, S.; Sullivan, E.; Karyekar, C.S.; Li, N. The multifaceted impact of anxiety and depression on patients with rheumatoid arthritis. BMC Rheumatol. 2019, 3, 43. [Google Scholar] [CrossRef] [Green Version]
- Bielefeld, P.; Saadoun, D.; Héron, E.; Abad, S.; Devilliers, H.; Deschasse, C.; Trad, S.; Sène, D.; Kaplanski, G.; Sève, P. Scleritis and systemic diseases: What should know the internist? Rev. Med. Interne 2018, 39, 711–720. [Google Scholar] [CrossRef]
- Yoshida, A.; Watanabe, M.; Okubo, A.; Kawashima, H. Clinical characteristics of scleritis patients with emphasized comparison of associated systemic diseases (anti-neutrophil cytoplasmic antibody-associated vasculitis and rheumatoid arthritis). Jpn. J. Ophthalmol. 2019, 63, 417–424. [Google Scholar] [CrossRef]
- Kobayashi, T.; Takai, N.; Tada, R.; Shoda, H.; Kida, T.; Ikeda, T.; Ozaki, T.; Makino, S. A case of scleritis associated rheumatoid arthritis accompanying an intraocular elevated lesion. BMC Ophthalmol. 2018, 18, 129. [Google Scholar] [CrossRef]
- Julian, L.J.; Yazdany, J.; Trupin, L.; Criswell, L.A.; Yelin, E.; Katz, P.P. Validity of brief screening tools for cognitive impairment in rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res. (Hoboken) 2012, 64, 448–454. [Google Scholar] [CrossRef] [Green Version]
- Muriel Deutsch Howieson, L.; Bigler, D.B.; Erin, D.; Tranel, D. Neuropsychological Assessment, 5th ed.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Ling, S.; Bluett, J.; Barton, A. Prediction of response to methotrexate in rheumatoid arthritis. Expert Rev. Clin. Immunol. 2018, 14, 419–429. [Google Scholar] [CrossRef]
- Gorelick, P.B. Role of inflammation in cognitive impairment: Results of observational epidemiological studies and clinical trials. Ann. N. Y. Acad. Sci. 2010, 1207, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Attal, N.; Masselin-Dubois, A.; Martinez, V.; Jayr, C.; Albi, A.; Fermanian, J.; Bouhassira, D.; Baudic, S. Does cognitive functioning predict chronic pain? Results from a prospective surgical cohort. Brain 2014, 137, 904–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.Y.; Katz, P.; Wallhagen, M.; Julian, L. Cognitive impairment in persons with rheumatoid arthritis. Arthritis Care Res. (Hoboken) 2012, 64, 1144–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favero, G.; Paganelli, C.; Buffoli, B.; Rodella, L.F.; Rezzani, R. Endothelium and its alterations in cardiovascular diseases: Life style intervention. BioMed Res. Int. 2014, 2014, 801896. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.R.; Wang, S.N.; Zhu, S.Y.; Wang, Y.Q.; Li, Z.Z.; Liu, Z.-Y.; Jiang, W.-S.; Chen, J.-T.; Wu, Q. Advanced oxidation protein products increase TNF-alpha and IL-1beta expression in chondrocytes via NADPH oxidase 4 and accelerate cartilage degeneration in osteoarthritis progression. Redox Biol. 2020, 28, 101306. [Google Scholar] [CrossRef]
- García-Vicuña, R.; Gómez-Gaviro, M.V.; Domínguez-Luis, M.J.; Pec, M.K.; González-Alvaro, I.; Alvaro-Gracia, J.M.; Díaz-González, F. CC and CXC chemokine receptors mediate migration, proliferation, and matrix metalloproteinase production by fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Rheum. 2004, 50, 3866–3877. [Google Scholar] [CrossRef]
- Abramson, S.B.; Amin, A. Blocking the effects of IL-1 in rheumatoid arthritis protects bone and cartilage. Rheumatology (Oxford) 2002, 41, 972–980. [Google Scholar] [CrossRef] [Green Version]
- Tornero Molina, J.; Balsa Criado, A.; Blanco García, F.; Blanco Alonso, R.; Bustabad, S.; Alen, J.C.; Corominas, H.; Nebro, A.F.; Ivorra, J.A.R.; Sanmartí, R. Expert Recommendations on the Interleukin 6 Blockade in Patients with Rheumatoid Arthritis. Reumatol. Clin. 2020, 16, 272–281. [Google Scholar] [CrossRef]
- Narazaki, M.; Tanaka, T.; Kishimoto, T. The role and therapeutic targeting of IL-6 in rheumatoid arthritis. Expert Rev. Clin. Immunol. 2017, 13, 535–551. [Google Scholar] [CrossRef]
- Rahmati, M.; Moosavi, M.A.; McDermott, M.F. ER Stress: A Therapeutic Target in Rheumatoid Arthritis? Trends Pharmacol Sci. 2018, 39, 610–623. [Google Scholar] [CrossRef]
- Wang, S.; Kaufman, R.J. The impact of the unfolded protein response on human disease. J. Cell Biol. 2012, 197, 857–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gargalovic, P.S.; Gharavi, N.M.; Clark, M.J.; Pagnon, J.; Yang, W.P.; He, A.; Truong, A.; Baruch-Oren, T.; Berliner, J.A.; Kirchgessner, T.G.; et al. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasnain, S.Z.; Lourie, R.; Das, I.; Chen, A.C.; McGuckin, M.A. The interplay between endoplasmic reticulum stress and inflammation. Immunol. Cell Biol. 2012, 90, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Fernandes, C.; Liu, Y.; Wu, Y.; Wu, H.; Brophy, M.L.; Deng, L.; Song, K.; Wen, A.; Wong, S.; et al. Role of endoplasmic reticulum stress signalling in diabetic endothelial dysfunction and atherosclerosis. Diab. Vasc. Dis. Res. 2017, 14, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.A.; Barnes, M.D.; Hong, D.; DeLay, M.L.; Inman, R.D.; Colbert, R.A. Gene expression analysis of macrophages derived from ankylosing spondylitis patients reveals interferon-gamma dysregulation. Arthritis Rheum. 2008, 58, 1640–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplon, R.E.; Chung, E.; Reese, L.; Cox-York, K.; Seals, D.R.; Gentile, C.L. Activation of the unfolded protein response in vascular endothelial cells of nondiabetic obese adults. J. Clin. Endocrinol. Metab. 2013, 98, E1505–E1509. [Google Scholar] [CrossRef] [Green Version]
- DeLay, M.L.; Turner, M.J.; Klenk, E.I.; Smith, J.A.; Sowders, D.P.; Colbert, R.A. HLAB27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 2009, 60, 2633–6643. [Google Scholar] [CrossRef]
- Todd, D.J.; Lee, A.H.; Glimcher, L.H. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat. Rev. Immunol. 2008, 8, 663–674. [Google Scholar] [CrossRef]
- Park, Y.J.; Yoo, S.A.; Kim, W.U. Role of endoplasmic reticulum stress in rheumatoid arthritis pathogenesis. J. Korean Med. Sci. 2014, 29, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Bailey, K.A.; Moreno, E.; Haj, F.G.; Simon, S.I.; Passerini, A.G. Mechanoregulation of p38 activity enhances endoplasmic reticulum stress-mediated inflammation by arterial endothelium. FASEB J. 2019, 33, 12888–12899. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb. Exp. Pharmacol. 2014, 220, 223–250. [Google Scholar] [PubMed]
- Lessmann, V.; Brigadski, T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: An update. Neurosci. Res. 2009, 65, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.G.; Irier, H.A.; Gu, J.; Tian, D.; Ku, L.; Liu, G.; Xia, M.; Fritsch, B.; Zheng, J.Q.; Dingledine, R.; et al. Distinct 3’UTRs differentially regulate activity-dependent translation of brain-derived neurotrophic factor (BDNF). Proc. Natl. Acad. Sci. USA 2010, 107, 15945–15950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marie, C.; Pedard, M.; Quirié, A.; Tessier, A.; Garnier, P.; Totoson, P.; Demougeot, C. Brain-derived neurotrophic factor secreted by the cerebral endothelium: A new actor of brain function? J. Cereb. Blood Flow Metab. 2018, 38, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Z.; Wang, P.; Guan, S.Y.; Li, H.M.; Leng, R.X.; Pan, H.-F.; Ye, D.Q. Decreased flow-mediated dilatation in patients with rheumatoid arthritis: A meta-analysis. Postgrad. Med. J. 2017, 93, 260–265. [Google Scholar] [CrossRef]
- Pedard, M.; Quirié, A.; Garnier, P.; Tessier, A.; Demougeot, C.; Marie, C. The Cerebral Brain-Derived Neurotrophic Factor Pathway, Either Neuronal or Endothelial, Is Impaired in Rats with Adjuvant-Induced Arthritis. Connection with Endothelial Dysfunction. Front. Phisiol. 2018, 8, 1125. [Google Scholar] [CrossRef] [Green Version]
- Arts, E.E.; Popa, C.D.; Den Broeder, A.A.; Donders, R.; Sandoo, A.; Toms, T.; Rollefstad, S.; Ikdahl, E.; Semb, A.G.; Kitas, G.D.; et al. Prediction of cardiovascular risk in rheumatoid arthritis: Performance of original and adapted SCORE algorithms. Ann. Rheum. Dis. 2016, 75, 674–680. [Google Scholar] [CrossRef]
- Myasoedova, E.; Chandran, A.; Ilhan, B.; Major, B.T.; Michet, C.J.; Matteson, E.L.; Crowson, C.S. The role of rheumatoid arthritis (RA) flare and cumulative burden of RA severity in the risk of cardiovascular disease. Ann. Rheum. Dis. 2016, 75, 560–565. [Google Scholar] [CrossRef] [Green Version]
- Inaba, Y.; Chen, J.A.; Bergmann, S.R. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: A meta-analysis. Int. J. Cardiovasc. Imaging 2010, 26, 631–640. [Google Scholar] [CrossRef]
- Salvemini, D.; Kim, S.F.; Mollace, V. Reciprocal regulation of the nitric oxide and cyclooxygenase pathway in pathophysiology: Relevance and clinical implications. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, 473–487. [Google Scholar] [CrossRef] [Green Version]
- Mollace, V.; Muscoli, C.; Masini, E.; Cuzzocrea, S.; Salvemini, D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol. Rev. 2005, 57, 217–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- England, B.R.; Thiele, G.M.; Anderson, D.R.; Mikuls, T.R. Increased cardiovascular risk in rheumatoid arthritis: Mechanisms and implications. BMJ 2018, 361, k1036. [Google Scholar] [CrossRef] [PubMed]
- Monnier, A.; Prigent-Tessier, A.; Quirié, A.; Bertrand, N.; Savary, S.; Gondcaille, C.; Garnier, P.; Demougeot, C.; Marie, C. Brain-derived neurotrophic factor of the cerebral microvasculature: A forgotten and nitric oxide-dependent contributor of brain-derived neurotrophic factor in the brain. Acta Physiol. (Oxford) 2017, 219, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Shilkina, N.P.; Yunonin, I.E.; Butusova, S.V.; Mikhailova, E.V.; Vinogradov, A.A. Endothelial damage and circadian blood pressure profile in rheumatoid arthritis. Ter. Arkh. 2019, 91, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Atehortúa, L.; Rojas, M.; Vásquez, G.; Muñoz-Vahos, C.H.; Vanegas-García, A.; Posada-Duque, R.A.; Castaño, D. Endothelial activation and injury by microparticles in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 34. [Google Scholar] [CrossRef] [Green Version]
- Fenton, S.A.M.; Sandoo, A.; Metsios, G.S.; Duda, J.L.; Kitas, G.D.; Veldhuijzen van Zanten, J.J.C.S. Sitting time is negatively related to microvascular endothelium-dependent function in rheumatoid arthritis. Microvasc. Res. 2018, 117, 57–60. [Google Scholar] [CrossRef]
- Kim, G.W.; Lee, N.R.; Pi, R.H.; Lim, Y.S.; Lee, Y.M. IL-6 inhibitors for treatment of rheumatoid arthritis: Past, present, and future. Arch. Pharm. Res. 2015, 38, 575–584. [Google Scholar] [CrossRef]
- Choy, E.H.S.; Calabrese, L.H. Neuroendocrine and neurophysiological effects of interleukin 6 in rheumatoid arthritis. Rheumatology (Oxford) 2018, 57, 1885–1895. [Google Scholar] [CrossRef] [Green Version]
- Totoson, P.; Maguin-Gaté, K.; Nappey, M.; Wendling, D.; Demougeot, C. Endothelial Dysfunction in Rheumatoid Arthritis: Mechanistic Insights and Correlation with Circulating Markers of Systemic Inflammation. PLoS ONE 2016, 11, e014674. [Google Scholar] [CrossRef] [Green Version]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Carresi, C.; Scarano, F.; Bosco, F.; Nucera, S.; Ruga, S.; Zito, M.C.; et al. The “Frail” Brain Blood Barrier in Neurodegenerative Diseases: Role of Early Disruption of Endothelial Cell-to-Cell Connections. Int. J. Mol. Sci. 2018, 19, 2693. [Google Scholar] [CrossRef] [Green Version]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Macrì, R.; Scicchitano, M.; Bosco, F.; Scarano, F.; Ruga, S.; et al. The Role of Endothelial Dysfunction in Peripheral Blood Nerve Barrier: Molecular Mechanisms and Pathophysiological Implications. Int. J. Mol. Sci. 2019, 20, 3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamoke, F.; Mazzone, V.; Persichini, T.; Maraschi, A.; Harris, M.B.; Venema, R.C.; Colasanti, M.; Gliozzi, M.; Muscoli, C.; Bartoli, M.; et al. Amyloid β peptide-induced inhibition of endothelial nitric oxide production involves oxidative stress-mediated constitutive eNOS/HSP90 interaction and disruption of agonist-mediated Akt activation. J. Neuroinflamm. 2015, 12, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollace, V.; Gliozzi, M.; Musolino, V.; Carresi, C.; Muscoli, S.; Mollace, R.; Tavernese, A.; Gratteri, S.; Palma, E.; Morabito, C. Oxidized LDL attenuates protective autophagy and induces apoptotic cell death of endothelial cells: Role of oxidative stress and LOX-1 receptor expression. Int. J. Cardiol. 2015, 184, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Su, Y. Regulation of endothelial nitric oxide synthase activity by protein-protein interaction. Curr. Pharm. Des. 2014, 20, 3514–3520. [Google Scholar] [CrossRef] [PubMed]
- Mollace, V.; Gliozzi, M. The potential role of TLR4/caveolin-1/NOS pathway in oxyLDL-modulation of autophagic/apoptotic responses in endothelial cells. Int. J. Cardiol. 2016, 203, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Muscoli, C.; Cuzzocrea, S.; Riley, D.P.; Zweier, J.L.; Thiemermann, C.; Wang, Z.-Q.; Salvemini, D. On the selectivity of superoxide dismutase mimetics andits importance in pharmacological studies. Br. J. Pharmacol. 2003, 140, 445–460. [Google Scholar] [CrossRef] [Green Version]
- Salvemini, D.; Muscoli, C.; Riley, D.P.; Cuzzocrea, S. Superoxide dismutase mimetics. Pulm. Pharmacol. Ther. 2002, 15, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Nasriati, F.; Hidayat, R.; Budiman, B.; Rinaldi, I. Correlation Between Tumor Necrosis Factor-α Levels, Free Fatty Acid Levels, and Soluble Vascular Cell Adhesion Molecule-1 Levels in Rheumatoid Arthritis Patients. Open Rheumatol. J. 2018, 12, 86–93. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Porreca, F.; Cuzzocrea, S.; Galen, K.; Lightfoot, R.; Masini, E.; Muscoli, C.; Mollace, V.; Ndengele, M.; Ischiropoulos, H.; et al. A newly identified role for superoxide in inflammatory pain. J. Pharmacol. Exp. Ther. 2004, 309, 869–878. [Google Scholar] [CrossRef]
- Muscoli, C.; Dagostino, C.; Ilari, S.; Lauro, F.; Gliozzi, M.; Bardhi, E.; Palma, E.; Mollace, V.; Salvemini, D. Posttranslational nitration of tyrosine residues modulates glutamate transmission and contributes to N-methyl-D-aspartate-mediated thermal hyperalgesia. Mediat. Inflamm. 2013, 2013, 950947. [Google Scholar] [CrossRef] [Green Version]
- El, M.; Ashour, S.; Moustafa, H.; Ahmed, I. Altered levels of soluble adhesion molecules in patients with rheumatoid arthritis complicated by peripheral neuropathy. J. Rheumatol. 2002, 29, 57–61. [Google Scholar] [PubMed]
- Luchetti, F.; Crinelli, R.; Cesarini, E.; Canonico, B.; Guidi, L.; Zerbinati, C.; Di Sario, G.; Zamai, L.; Magnani, M.; Papa, S.; et al. Endothelial cells, endoplasmic reticulum stress and oxysterols. Redox Biol. 2017, 13, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Zhenyukh, O.; González-Amor, M.; Rodrigues-Diez, R.R.; Esteban, V.; Ruiz-Ortega, M.; Salaices, M.; Mas, S.; Briones, A.M.; Egido, J. Branched-chain amino acids promote endothelial dysfunction through increased reactive oxygen species generation and inflammation. J. Cell Mol. Med. 2018, 22, 4948–4962. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Navarra, M.; Maiuolo, J.; Rotiroti, D.; Bagetta, G.; Corasaniti, M.T. 17beta-estradiol protects SH-SY5Y Cells against HIV-1 gp120-induced cell death: Evidence for a role of estrogen receptors. Neurotoxicology 2005, 26, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, B.O. Modulation of the inflammatory response by estrogens with focus on the endothelium and its interactions with leukocytes. Inflamm. Res. 2007, 56, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Ortona, E.; Pierdominici, M.; Maselli, A.; Veroni, C.; Aloisi, F.; Shoenfeld, Y. Sex-based differences in autoimmune diseases. Ann. Ist. Super Sanita 2016, 52, 205–212. [Google Scholar]
- Yasuda, H.; Sonoda, A.; Yamamotom, M.; Kawashima, Y.; Takishita, Y.; Morita, A.; Tsutsumi, T.; Tsuchiya, M.; Sato, E.F. 17-β-estradiol enhances neutrophil extracellular trap formation by interaction with estrogen membrane receptor. Arch. Biochem. Biophys. 2019, 663, 64–70. [Google Scholar] [CrossRef]
- Manuel, D.G.; Perez, R.; Sanmartin, C.; Taljaard, M.; Hennessy, D.; Wilson, K.; Tanuseputro, P.; Manson, H.; Bennett, C.; Tuna, M.; et al. Measuring Burden of Unhealthy Behaviours Using a Multivariable Predictive Approach: Life Expectancy Lost in Canada Attributable to Smoking, Alcohol, Physical Inactivity, and Diet. PLoS Med. 2016, 13, e1002082. [Google Scholar] [CrossRef]
- O’Doherty, M.G.; Cairns, K.; O’Neill, V.; Lamrock, F.; Jørgensen, T.; Brenner, H.; Schöttker, B.; Wilsgaard, T.; Siganos, G.; Kuulasmaa, K.; et al. Effect of major lifestyle risk factors, independent and jointly, on life expectancy with and without cardiovascular disease: Results from the Consortium on Health and Ageing Network of Cohorts in Europe and the United States (CHANCES). Eur. J. Epidemiol. 2016, 31, 455–468. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Pan, A.; Wang, D.D.; Liu, X.; Dhana, K.; Franco, O.H.; Kaptoge, S.; Di Angelantonio, E.; Stampfer, M.; Willett, W.C.; et al. Impact of Healthy Lifestyle Factors on Life Expectancies in the US Population. Circulation 2018, 138, 345–355. [Google Scholar] [CrossRef]
- Gwinnutt, J.M.; Verstappen, S.M.; Humphreys, J.H. The impact of lifestyle behaviours, physical activity and smoking on morbidity and mortality in patients with rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101562. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, D.; Discacciati, A.; Orsini, N.; Wolk, A. Cigarette smoking and risk of rheumatoid arthritis: A dose-response meta-analysis. Arthritis Res. Ther. 2014, 16, R61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myasoedova, E.; Davis, J.; Matteson, E.L.; Crowson, C.S. Is the epidemiology of rheumatoid arthritis changing? Results from a population-based incidence study, 1985–2014. Ann. Rheum. Dis. 2020. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Rausch Osthoff, A.K.; Juhl, C.B.; Knittle, K.; Braun, J.; Schoones, J.; Vliet Vlieland, T.P.M.; Niedermann, K. Effects of exercise and physical activity promotion: Meta-analysis informing the 2018 EULAR recommendations for physical activity in people with rheumatoid arthritis, spondyloarthritis and hip/knee osteoarthritis. RMD Open 2018, 4, e000713. [Google Scholar] [CrossRef]
- Larsson, I.; Andersson, M.L.E. Reasons to stop drinking alcohol among patients with rheumatoid arthritis in Sweden: A mixed-methods study. BMJ Open 2018, 8, e024367. [Google Scholar] [CrossRef] [Green Version]
- Maurel, D.B.; Boisseau, N.; Benhamou, C.L.; Jaffre, C. Alcohol and bone: Review of dose effects and mechanisms. Osteoporos. Int. 2012, 23, 1–16. [Google Scholar] [CrossRef]
- Schmidt, R.E.; Grimbacher, B.; Witte, T. Autoimmunity and primary immunodeficiency: Two sides of the same coin? Nat. Rev. Rheumatol. 2017, 14, 7–18. [Google Scholar] [CrossRef]
- Tedeschi, S.K.; Frits, M.; Cui, J.; Zhang, Z.; Mahmoud, T.; Lin, T.C.; Yoshida, K.; Weinblatt, M.E.; Shadick, N.A.; Solomon, D.H. Diet and Rheumatoid Arthritis Symptoms: Survey Results from a Rheumatoid Arthritis Registry. Arthritis Care Res. (Hoboken) 2017, 69, 1920–1925. [Google Scholar] [CrossRef] [Green Version]
- Sousa Guerreiro, C.; Calado, A.; Sousa, J.; Fonseca, J.E. Diet, Microbiota, and Gut Permeability The Unknown Triad in Rheumatoid Arthritis. Front. Med. 2018, 5, 349. [Google Scholar] [CrossRef] [Green Version]
- De Gregori, M.; Muscoli, C.; Schatman, M.E.; Stallone, T.; Intelligente, F.; Rondanelli, M.; Franceschi, F.; Arranz, L.I.; Lorente-Cebrián, S.; Salamone, M.; et al. Combining pain therapy with lifestyle: The role of personalized nutrition and nutritional supplements according to the SIMPAR Feed Your Destiny approach. J. Pain Res. 2016, 9, 1179–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Gregori, M.; Belfer, I.; De Giorgio, R.; Marchesini, M.; Muscoli, C.; Rondanelli, M.; Martini, D.; Mena, P.; Arranz, L.I.; Lorente-Cebrián, S.; et al. Second edition of SIMPAR’s “Feed Your Destiny” workshop: The role of lifestyle in improving pain management. J. Pain Res. 2018, 11, 1627–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, K.; Askling, J.; Alfredsson, L.; Di Giuseppe, D. Mediterranean diet and risk of rheumatoid arthritis: A population-based case-control study. EIRA study group. Arthritis Res Ther. 2018, 20, 175. [Google Scholar] [CrossRef] [PubMed]
- Philippou, E.; Nikiphorou, E. Are we really what we eat? Nutrition and its role in the onset of rheumatoid arthritis. Autoimmun. Rev. 2018, 17, 1074–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Garcia, E.; Hu, F.B. Nutrition and the endothelium. Curr. Diab. Rep. 2004, 4, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.R.; Hodgson, J.M.; Woodman, R.; Bryan, J.; Wilson, C.; Murphy, K.J. A Mediterranean diet lowers blood pressure and improves endothelial function: Results from the MedLey randomized intervention trial. Am. J. Clin. Nutr. 2017, 105, 1305–1313. [Google Scholar] [CrossRef] [Green Version]
- Atzeni, F.; Talotta, R.; Masala, I.; Gerardi, M.C.; Casale, R.; Sarzi-Puttini, P. Central nervous system involvement in rheumatoid arthritis patients and the potential implications of using biological agents. Best Pract. Res. Clin. Rheumatol. 2018, 32, 500–510. [Google Scholar] [CrossRef]
- Dudics, S.; Langan, D.; Meka, R.R.; Venkatesha, S.H.; Berman, B.M.; Berman, B.M.; Che, C.-T.; Moudgil, K.D. Natural Products for the Treatment of Autoimmune Arthritis: Their Mechanisms of Action, Targeted Delivery, and Interplay with the Host Microbiome. Int. J. Mol. Sci. 2018, 19, 2508. [Google Scholar] [CrossRef] [Green Version]
- Rosillo, M.A.; Alarcón-de-la-Lastra, C.; Sánchez-Hidalgo, M. An update on dietary phenolic compounds in the prevention and management of rheumatoid arthritis. Food Funct. 2016, 7, 2943–2969. [Google Scholar] [CrossRef]
- Lauro, F.; Giancotti, L.A.; Ilari, S.; Dagostino, C.; Gliozzi, M.; Morabito, C.; Malafoglia, V.; Raffaeli, W.; Muraca, M.; Goffredo, B.M.; et al. Inhibition of Spinal Oxidative Stress by Bergamot Polyphenolic Fraction Attenuates the Development of Morphine Induced Tolerance and Hyperalgesia in Mice. PLoS ONE 2016, 11, e0156039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilari, S.; Giancotti, L.A.; Lauro, F.; Dagostino, C.; Gliozzi, M.; Malafoglia, V.; Sansone, L.; Palma, E.; Tafani, M.; Russo, M.A.; et al. Antioxidant modulation of sirtuin 3 during acute inflammatory pain: The ROS control. Pharmacol. Res. 2020, 157, 104851. [Google Scholar] [CrossRef] [PubMed]
- Muscoli, C.; Lauro, F.; Dagostino, C.; Ilari, S.; Giancotti, L.A.; Gliozzi, M.; Costa, N.; Carresi, C.; Musolino, V.; Casale, F.; et al. Olea Europea-derived phenolic products attenuate antinociceptive morphine tolerance: An innovative strategic approach to treat cancer pain. J. Biol. Regul. Homeost. Agents 2014, 28, 105–116. [Google Scholar] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oppedisano, F.; Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Scicchitano, M.; Scarano, F.; Bosco, F.; Macrì, R.; et al. The Potential for Natural Antioxidant Supplementation in the Early Stages of Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 2618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.Z.; Zhang, S.N. Herbal compounds for rheumatoid arthritis: Literatures review and cheminformatics prediction. Phytother. Res. 2020, 34, 51–66. [Google Scholar] [CrossRef]
- Yang, G.; Chang, C.C.; Yang, Y.; Yuan, L.; Xu, L.; Ho, C.T.; Li, S. Resveratrol Alleviates Rheumatoid Arthritis via Reducing ROS and Inflammation, Inhibiting MAPK Signaling Pathways, and Suppressing Angiogenesis. J. Agric. Food Chem. 2018, 66, 12953–12960. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, G.; Wang, T.; Cao, W.; Zhang, L.; Chen, X. Nrf2-Keap1 pathway-mediated effects of resveratrol on oxidative stress and apoptosis in hydrogen peroxide-treated rheumatoid arthritis fibroblast-like synoviocytes. Ann. N. Y. Acad. Sci. 2019, 1457, 166–178. [Google Scholar] [CrossRef]
- Khojah, H.M.; Ahmed, S.; Abdel-Rahman, M.S.; Elhakeim, E.H. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: A clinical study. Clin. Rheumatol. 2018, 37, 2035–2042. [Google Scholar] [CrossRef]
- Santangelo, C.; Vari, R.; Scazzocchio, B.; De Sanctis, P.; Giovannini, C.; D’Archivio, M.; Masella, R. Anti-inflammatory Activity of Extra Virgin Olive Oil Polyphenols: Which Role in the Prevention and Treatment of Immune-Mediated Inflammatory Diseases? Endocr. Metab Immune Disord. Drug Targets 2018, 18, 36–50. [Google Scholar] [CrossRef]
- Johansson, D.; Mortaji, S.; Rezaei, M.; Shaikhi, M. The Effectiveness of Olive Oil in Controlling Morning Inflammatory Pain of Phalanges and Knees Among Women With Rheumatoid Arthritis: A Randomized Clinical Trial. Rehabil. Nurs. 2020, 45, 106–113. [Google Scholar]
- Quintero-Flórez, A.; Pereira-Caro, G.; Sánchez-Quezada, C.; Moreno-Rojas, J.M.; Gaforio, J.J.; Jimenez, A.; Beltrán, G. Effect of olive cultivar on bioaccessibility and antioxidant activity of phenolic fraction of virgin olive oil. Eur. J. Nutr. 2018, 57, 1925–1946. [Google Scholar]
- Bulotta, S.; Corradino, R.; Celano, M.; Maiuolo, J.; D’Agostino, M.; Oliverio, M.; Procopio, A.; Filetti, S.; Russo, D. Antioxidant and antigrowth action of peracetylated oleuropein in thyroid cancer cells. J. Mol. Endocrinol. 2013, 51, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angeloni, C.; Malaguti, M.; Barbalace, M.C.; Hrelia, S. Bioactivity of Olive Oil Phenols in Neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230. [Google Scholar] [CrossRef] [Green Version]
- Castejón, M.L.; Rosillo, M.Á.; Montoya, T.; González-Benjumea, A.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Oleuropein down-regulated IL-1β-induced inflammation and oxidative stress in human synovial fibroblast cell line SW982. Food Funct. 2017, 8, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Kwon, D.; Um, E.; Kim, B. Could Polyphenols Help in the Control of Rheumatoid Arthritis? Molecules 2019, 24, 1589. [Google Scholar] [CrossRef] [Green Version]
- Bonacci, S.; Paonessa, R.; Costanzo, P.; Salerno, R.; Maiuolo, J.; Nardi, M.; Procopio, A.; Oliverio, M. Peracetylation as a strategy to improve oleuropein stability and its affinity to fatty foods. Food Funct. 2018, 9, 5759–5767. [Google Scholar] [CrossRef]
- Yang, M.; Akbar, U.; Mohan, C. Curcumin in Autoimmune and Rheumatic Diseases. Nutrients 2019, 11, 1004. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.; Sepodes, B.; Rocha, J.; Direito, R.; Fernandes, A.; Brites, D.; Freitas, M.; Fernandes, E.; Bronze, M.R.; Figueira, M. Protective effects of hydroxytyrosol-supplemented refined olive oil in animal models of acute inflammation and rheumatoid arthritis. J. Nutr. Biochem. 2015, 26, 360–368. [Google Scholar] [CrossRef]
- Aparicio-Soto, M.; Sánchez-Hidalgo, M.; Rosillo, M.Á.; Castejón, M.L.; Alarcón-de-la-Lastra, C. Extra virgin olive oil: A key functional food for prevention of immune-inflammatory diseases. Food Funct. 2016, 7, 4492–4505. [Google Scholar] [CrossRef]
- Dai, Q.; Zhou, D.; Xu, L.; Song, X. Curcumin alleviates rheumatoid arthritis-induced inflammation and synovial hyperplasia by targeting mTOR pathway in rats. Drug Des. Devel. Ther. 2018, 12, 4095–4105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Scarano, F.; Bosco, F.; Nucera, S.; Maiuolo, J.; Macrì, R.; Ruga, S.; et al. The Effect of Natural Antioxidants in the Development of Metabolic Syndrome: Focus on Bergamot Polyphenolic Fraction. Nutrients 2020, 12, 1504. [Google Scholar] [CrossRef] [PubMed]
- Musolino, V.; Gliozzi, M.; Bombardelli, E.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; et al. The synergistic effect of Citrus bergamia and Cynara cardunculus extracts on vascular inflammation and oxidative stress in non-alcoholic fatty liver disease. J. Tradit. Complement. Med. 2020, 10, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Perna, S.; Spadaccini, D.; Botteri, L.; Girometta, C.; Riva, A. Efficacy of bergamot: From anti-inflammatory and anti-oxidative mechanisms to clinical applications as preventive agent for cardiovascular morbidity, skin diseases, and mood alterations. Food Sci. Nutr. 2019, 7, 369–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Impellizzeri, D.; Bruschetta, G.; Di Paola, R.; Ahmad, A.; Campolo, M. The anti-inflammatory and antioxidant effects of bergamot juice extract (BJe) in an experimental model of inflammatory bowel disease. Clin. Nutr. 2015, 34, 1146–1154. [Google Scholar] [CrossRef]
- Mollace, V.; Scicchitano, M.; Paone, S.; Casale, F.; Calandruccio, C.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Nucera, S.; et al. Hypoglycemic and Hypolipemic Effects of a New Lecithin Formulation of Bergamot Polyphenolic Fraction: A Double Blind, Randomized, Placebo- Controlled Study. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 136–143. [Google Scholar] [CrossRef]
- Rosell, M.; Wesley, A.M.; Rydin, K.; Klareskog, L.; Alfredsson, L. Dietary fish and fish oil and the risk of rheumatoid arthritis. EIRA study group. Epidemiology 2009, 20, 896–901. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiuolo, J.; Muscoli, C.; Gliozzi, M.; Musolino, V.; Carresi, C.; Paone, S.; Ilari, S.; Mollace, R.; Palma, E.; Mollace, V. Endothelial Dysfunction and Extra-Articular Neurological Manifestations in Rheumatoid Arthritis. Biomolecules 2021, 11, 81. https://doi.org/10.3390/biom11010081
Maiuolo J, Muscoli C, Gliozzi M, Musolino V, Carresi C, Paone S, Ilari S, Mollace R, Palma E, Mollace V. Endothelial Dysfunction and Extra-Articular Neurological Manifestations in Rheumatoid Arthritis. Biomolecules. 2021; 11(1):81. https://doi.org/10.3390/biom11010081
Chicago/Turabian StyleMaiuolo, Jessica, Carolina Muscoli, Micaela Gliozzi, Vincenzo Musolino, Cristina Carresi, Sara Paone, Sara Ilari, Rocco Mollace, Ernesto Palma, and Vincenzo Mollace. 2021. "Endothelial Dysfunction and Extra-Articular Neurological Manifestations in Rheumatoid Arthritis" Biomolecules 11, no. 1: 81. https://doi.org/10.3390/biom11010081