5-Hydroxytryptamine (5-HT) Positively Regulates Pigmentation via Inducing Melanoblast Specification and Melanin Synthesis in Zebrafish Embryos
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zebrafish Maintenance and Ethic Statements
2.2. Drug Treatment
2.3. Whole-Mount in situ Hybridization
2.4. RNA Extraction and PCR
2.5. Measurement of the Pigmenting Activity in the Zebrafish
2.6. Melanin Content and Tyrosinase Activity Assay
2.7. Cell Culture
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
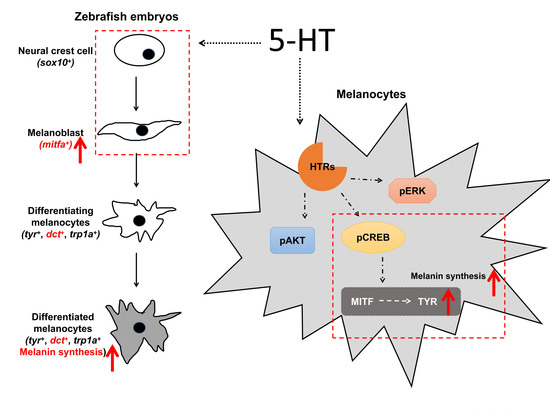
3.1. 5-HT Induces Pigmentation in a Dose Dependent Manner in Zebrafish Embryos
3.2. 5-HT Promotes the Processes of both Melanocytes Development and Melanin Synthesis
3.3. 5-HT Facilitate the Melanoblast Specification from Neural Crest Cells
3.4. 5-HT Prompts Regeneration of Larval Zebrafish Melanocytes
3.5. 5-HT Increases the Expression of MITF and TYR by Activating the PKA/p-CREB Signaling in Melanoma Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Tobin, D.; Shibahara, S.; Wortsman, J. Melanin Pigmentation in Mammalian Skin and Its Hormonal Regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Mort, R.L.; Jackson, I.J.; Patton, E.E. The melanocyte lineage in development and disease. Development 2015, 142, 1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawakami, A.; Fisher, D.E. Key Discoveries in Melanocyte Development. J. Investig. Dermatol. 2011, 131, E2–E4. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; Arnheiter, H.; Pavan, W.J. Interspecies difference in the regulation of melanocyte development by SOX10 and MITF. Proc. Natl. Acad. Sci. USA 2006, 103, 9081–9085. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; Pavan, W.J. Transcriptional and signaling regulation in neural crest stem cell-derived melanocyte development: Do all roads lead to Mitf? Cell Res. 2008, 18, 1163–1176. [Google Scholar] [CrossRef]
- Fang, D.; Kute, T.; Setaluri, V. Regulation of Tyrosinase-related Protein-2 (TYRP2) in Human Melanocytes: Relationship to Growth and Morphology. Pigment. Cell Res. 2001, 14, 132–139. [Google Scholar] [CrossRef]
- Chen, L.; Ren, X.; Liang, F.; Li, S.; Zhong, H.; Lin, S. Characterization of two novel small molecules targeting melanocyte development in zebrafish embryogenesis. Pigment. Cell Melanoma Res. 2012, 25, 446–453. [Google Scholar] [CrossRef]
- Choi, T.-Y.; Kim, J.-H.; Ko, D.H.; Kim, C.-H.; Hwang, J.-S.; Ahn, S.; Kim, S.Y.; Kim, C.D.; Lee, J.-H.; Yoon, T.-J. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment. Cell Res. 2007, 20, 120–127. [Google Scholar] [CrossRef]
- Kelsh, R.; Schmid, B.; Eisen, J.S. Genetic Analysis of Melanophore Development in Zebrafish Embryos. Dev. Biol. 2000, 225, 277–293. [Google Scholar] [CrossRef]
- Camp, E.; Lardelli, M. Tyrosinase gene expression in zebrafish embryos. Dev. Genes Evol. 2001, 211, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Kasheta, M.; Ceol, C. Poised Regeneration of Zebrafish Melanocytes Involves Direct Differentiation and Concurrent Replenishment of Tissue-Resident Progenitor Cells. Dev. Cell 2015, 33, 631–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tryon, R.C.; Higdon, C.W.; Johnson, S.L. Lineage Relationship of Direct-Developing Melanocytes and Melanocyte Stem Cells in the Zebrafish. PLoS ONE 2011, 6, e21010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellgren, E.M.; Johnson, S.L. Kitb, a Second Zebrafish Ortholog of Mouse Kit. Dev. Genes Evol. 2005, 215, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Rawls, J.F.; Johnson, S.L. Zebrafish kit mutation reveals primary and secondary regulation of melanocyte development during fin stripe regeneration. Development 2000, 127, 3715–3724. [Google Scholar] [PubMed]
- Lee, H.; Park, M.; Kim, S.; Park Choo, H.Y.; Lee, A.; Lee, C. Serotonin induces melanogenesis via serotonin receptor 2A. Br. J. Dermatol. 2011, 165, 1344–1348. [Google Scholar] [CrossRef]
- Zhou, L.; Cai, M.; Ren, Y.; Wu, H.; Liu, M.; Chen, H.; Shang, J. The different roles of 5-HT1A/2A receptors in fluoxetine ameliorated pigmentation of C57BL/6 mouse skin in response to stress. J. Dermatol. Sci. 2018, 92, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.; Shang, J.; Tian, X.; Fan, X.; Shi, X.; Pei, S.; Wang, Q.; Yu, B.-Y. Up-regulation of melanin synthesis by the antidepressant fluoxetine. Exp. Dermatol. 2012, 21, 635–637. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, Y.; Huang, Q.; Cai, M.; Pan, Q.; Fu, M.; An, X.; Xia, Z.; Liu, M.; Jin, Y.; et al. NK1R/5-HT1AR interaction is related to the regulation of melanogenesis. FASEB J. 2018, 32, 3193–3214. [Google Scholar] [CrossRef] [Green Version]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Yang, C.-T.; Johnson, S.L. Small molecule-induced ablation and subsequent regeneration of larval zebrafish melanocytes. Development 2006, 133, 3563–3573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Li, K.; Dong, X.; Liang, D.; Zhao, Q. Ncor1 and Ncor2 play essential but distinct roles in zebrafish primitive myelopoiesis. Dev. Dyn. 2014, 243, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Beirl, A.J.; Linbo, T.H.; Cobb, M.J.; Cooper, C.D. oca2 regulation of chromatophore differentiation and number is cell type specific in zebrafish. Pigment. Cell Melanoma Res. 2014, 27, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.A.; Marie, K.L.; Lu, Y.; Brombin, A.; Santoriello, C.; Zeng, Z.; Zich, J.; Gautier, P.; Von Kriegsheim, A.; Brunsdon, H.; et al. PRL3-DDX21 Transcriptional Control of Endolysosomal Genes Restricts Melanocyte Stem Cell Differentiation. Dev. Cell 2020, 54, 317–332.e9. [Google Scholar] [CrossRef]
- Arck, P.C.; Slominski, A.T.; Theoharides, T.C.; Peters, E.M.J.; Paus, R. Neuroimmunology of Stress: Skin Takes Center Stage. J. Investig. Dermatol. 2006, 126, 1697–1704. [Google Scholar] [CrossRef] [Green Version]
- Nordlind, K.; Azmitia, E.C.; Slominski, A.T. The skin as a mirror of the soul: Exploring the possible roles of serotonin. Exp. Dermatol. 2008, 17, 301–311. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, H.; Zhou, J.; Pei, S.; Li, J.; Cai, Y.; Shang, J. Involvement of the central hypothalamic-pituitary-adrenal axis in hair growth and melanogenesis among different mouse strains. PLoS ONE 2018, 13, e0202955. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.T.; Pisarchik, A.; Wortsman, J. Expression of genes coding melatonin and serotonin receptors in rodent skin. Biochim. Biophys. Acta BBA 2004, 1680, 67–70. [Google Scholar] [CrossRef]
- Slominski, A.T.; Pisarchik, A.; Johansson, O.; Jing, C.; Semak, I.; Slugocki, G.; Wortsman, J. Tryptophan hydroxylase expression in human skin cells. Biochim. Biophys. Acta BBA 2003, 1639, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Hultman, K.A.; Johnson, S.L. Differential contribution of direct-developing and stem cell-derived melanocytes to the zebrafish larval pigment pattern. Dev. Biol. 2010, 337, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Gramann, A.K.; Venkatesan, A.M.; Guerin, M.; Ceol, C. Regulation of zebrafish melanocyte development by ligand-dependent BMP signaling. eLife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Tsai, R.-K.; Tsai, M.-H.; Lin, Y.-H.; Hirobe, T. The roles of Frizzled-3 and Wnt3a on melanocyte development: In vitro studies on neural crest cells and melanocyte precursor cell lines. J. Dermatol. Sci. 2014, 75, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.-Y.; Noh, M. The regulation of epidermal melanogenesis via cAMP and/or PKC signaling pathways: Insights for the development of hypopigmenting agents. Arch. Pharmacal Res. 2013, 36, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-M.; Kim, M.Y.; Sohn, K.-C.; Jung, S.-Y.; Lee, H.-E.; Lim, J.W.; Kim, S.; Lee, Y.; Im, M.; Seo, Y.-J.; et al. Nrf2 Negatively Regulates Melanogenesis by Modulating PI3K/Akt Signaling. PLoS ONE 2014, 9, e96035. [Google Scholar] [CrossRef] [Green Version]
- Rzepka, Z.; Buszman, E.; Beberok, A.; Wrześniok, D. From tyrosine to melanin: Signaling pathways and factors regulating melanogenesis. Postępy Hig. Med. Doświadczalnej 2016, 70, 695–708. [Google Scholar] [CrossRef]
- Lee, H.-E.; Song, J.; Kim, S.Y.; Park, K.-C.; Min, K.-H.; Kim, D.-S. MMS 1001 inhibits melanin synthesis via ERK activation. Die Pharm. 2013, 68, 212–216. [Google Scholar]
- Kim, D.-S.; Park, S.-H.; Kwon, S.-B.; Park, E.-S.; Huh, C.-H.; Youn, S.W.; Park, K.-C. Sphingosylphosphorylcholine-induced ERK activation inhibits melanin synthesis in human melanocytes. Pigment. Cell Res. 2006, 19, 146–153. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zhong, M.; Dong, J.; Chen, M.; Shang, J.; Yue, Y. 5-Hydroxytryptamine (5-HT) Positively Regulates Pigmentation via Inducing Melanoblast Specification and Melanin Synthesis in Zebrafish Embryos. Biomolecules 2020, 10, 1344. https://doi.org/10.3390/biom10091344
Liu L, Zhong M, Dong J, Chen M, Shang J, Yue Y. 5-Hydroxytryptamine (5-HT) Positively Regulates Pigmentation via Inducing Melanoblast Specification and Melanin Synthesis in Zebrafish Embryos. Biomolecules. 2020; 10(9):1344. https://doi.org/10.3390/biom10091344
Chicago/Turabian StyleLiu, Li, Min Zhong, Jing Dong, Minghan Chen, Jing Shang, and Yunyun Yue. 2020. "5-Hydroxytryptamine (5-HT) Positively Regulates Pigmentation via Inducing Melanoblast Specification and Melanin Synthesis in Zebrafish Embryos" Biomolecules 10, no. 9: 1344. https://doi.org/10.3390/biom10091344