Synthesis and Characterization of Oxidized Polysaccharides for In Situ Forming Hydrogels
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Oxidation of Polysaccharides
2.3. Characterization of Polysaccharides
2.3.1. Quantification of Aldehyde Groups in oPs
Schiff’s Test
Titration
2.3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.3. Molecular Weight Determination
2.4. Cytotoxicity Studies of Oxidized Polysaccharides
2.4.1. Cell Culture
2.4.2. Cytotoxicity Assay
2.4.3. Studies on Cell Viability and Morphology Using Vital Staining
2.5. Mechanical Characterization of Hydrogels by Rheometry
2.6. Cytotoxicity Studies of Hydrogels
2.6.1. Cell Culture
2.6.2. Cytotoxicity Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of Oxidized Polysaccharides
3.2. Cytotoxicity of Oxidized Alginate and Oxidized Hyaluronic Acid
3.3. Rheological Properties of oPs/CMC-Hydrogels
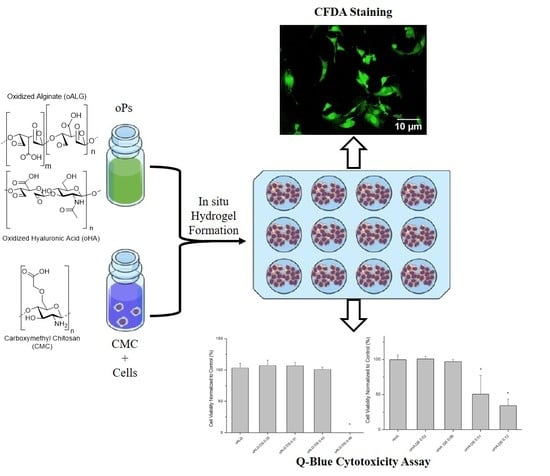
3.4. Cytotoxicity of Hydrogels Made of Oxidized Alginate and Oxidized Hyaluronic Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALG | alginate |
| CFDA | 5(6)-carboxyfluoresceine diacetate |
| CMC | carboxymethyl chitosan |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DOexp | experimental degree of oxidation |
| DSCHO | degree of substitution of aldehyde groups |
| EDTA | ethylenediamine tetraacetic acid |
| eq | equivalent |
| FBS | fetal bovine serum |
| FTIR | Fourier-transform infrared spectroscopy |
| G* | complex modulus |
| G’ | shear storage modulus |
| G” | loss modulus |
| GPC | gel permeation chromatography |
| HA | hyaluronic acid |
| HPLC | high performance liquid chromatography |
| ITS | insulin-transferrin-selenium A |
| Mw | weight average molecular weight |
| nALG | native alginate |
| nHA | native hyaluronic acid |
| oPs | oxidized polysaccharides |
| PBS | phosphate buffered saline |
| PEG | polyethylene glycol |
| Ps | polysaccharides |
| PSF | penicillin-streptomycin-fungizone |
| RT | room temperature |
| SD | standard deviations |
| UV/VIS spectroscopy | ultraviolet/visible spectroscopy |
References
- Ratner, B.D. Biomaterials: Been there, done that, and evolving into the future. Annu. Rev. Biomed. Eng. 2019, 21, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.M.; Papadimitrakopoulos, F.; Burgess, D.J. Biomaterials/tissue interactions: Possible solutions to overcome foreign body response. AAPS J. 2010, 12, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Domingues, R.M.; Silva, M.; Gershovich, P.; Betta, S.; Babo, P.; Caridade, S.G.; Mano, J.o.F.; Motta, A.; Reis, R.L.; Gomes, M.E. Development of injectable hyaluronic acid/cellulose nanocrystals bionanocomposite hydrogels for tissue engineering applications. Bioconjugate Chem. 2015, 26, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Pop-Georgievski, O.; Zimmermann, R.; Kotelnikov, I.; Proks, V.; Romeis, D.; Kučka, J.; Caspari, A.; Rypáček, F.E.; Werner, C. Impact of bioactive peptide motifs on molecular structure, charging, and nonfouling properties of poly (ethylene oxide) brushes. Langmuir 2018, 34, 6010–6020. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Mithieux, S.M.; Weiss, A.S. Elastin biomaterials in dermal repair. Trends Biotechnol. 2020, 38, 280–291. [Google Scholar] [CrossRef]
- Trujillo, S.; Gonzalez-Garcia, C.; Rico, P.; Reid, A.; Windmill, J.; Dalby, M.J.; Salmeron-Sanchez, M. Engineered 3D hydrogels with full-length fibronectin that sequester and present growth factors. Biomaterials 2020, 252, 120104. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Pradhan, S.; Agostinacchio, F.; Pal, R.K.; Greco, G.; Mazzolai, B.; Pugno, N.M.; Motta, A.; Yadavalli, V.K. Easy, scalable, robust, micropatterned silk fibroin cell substrates. Adv. Mater. Interfaces 2019, 6, 1801822. [Google Scholar] [CrossRef]
- Köwitsch, A.; Zhou, G.; Groth, T. Medical application of glycosaminoglycans: A review. J. Tissue Eng. Regen. Med. 2018, 12, e23–e41. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, Y.T.; Zeng, K.; Heinze, T.; Groth, T.; Zhang, K. Recent progress on cellulose-based ionic compounds for biomaterials. Adv. Mater. 2020, 2000717. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Grasdalen, H. High-field, 1H-nmr spectroscopy of alginate: Sequential structure and linkage conformations. Carbohydr. Res. 1983, 118, 255–260. [Google Scholar] [CrossRef]
- Criado-Gonzalez, M.; Fernandez-Gutierrez, M.; San Roman, J.; Mijangos, C.; Hernández, R. Local and controlled release of tamoxifen from multi (layer-by-layer) alginate/chitosan complex systems. Carbohydr. Polym. 2019, 206, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Kirdponpattara, S.; Khamkeaw, A.; Sanchavanakit, N.; Pavasant, P.; Phisalaphong, M. Structural modification and characterization of bacterial cellulose–alginate composite scaffolds for tissue engineering. Carbohydr. Polym. 2015, 132, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Price, R.D.; Berry, M.; Navsaria, H.A. Hyaluronic acid: The scientific and clinical evidence. J. Plast. Reconstr. Aesthetic Surg. 2007, 60, 1110–1119. [Google Scholar] [CrossRef]
- Kristiansen, K.A.; Potthast, A.; Christensen, B.E. Periodate oxidation of polysaccharides for modification of chemical and physical properties. Carbohydr. Res. 2010, 345, 1264–1271. [Google Scholar] [CrossRef]
- Millan, C.; Cavalli, E.; Groth, T.; Maniura-Weber, K.; Zenobi-Wong, M. Engineered microtissues formed by schiff base crosslinking restore the chondrogenic potential of aged mesenchymal stem cells. Adv. Healthc. Mater. 2015, 4, 1348–1358. [Google Scholar] [CrossRef] [Green Version]
- Reyes, J.M.; Herretes, S.; Pirouzmanesh, A.; Wang, D.-A.; Elisseeff, J.H.; Jun, A.; McDonnell, P.J.; Chuck, R.S.; Behrens, A. A modified chondroitin sulfate aldehyde adhesive for sealing corneal incisions. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1247–1250. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Van Tomme, S.R.; Storm, G.; Hennink, W.E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2008, 355, 1–18. [Google Scholar] [CrossRef]
- Mota, C.; Camarero-Espinosa, S.; Baker, M.B.; Wieringa, P.; Moroni, L. Bioprinting: From tissue and organ development to in vitro models. Chem. Rev. 2020. [Google Scholar] [CrossRef]
- Matyash, M.; Despang, F.; Ikonomidou, C.; Gelinsky, M. Swelling and mechanical properties of alginate hydrogels with respect to promotion of neural growth. Tissue Eng. Part C Methods 2014, 20, 401–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Segura, T.; Anderson, B.C.; Chung, P.H.; Webber, R.E.; Shull, K.R.; Shea, L.D. Crosslinked hyaluronic acid hydrogels: A strategy to functionalize and pattern. Biomaterials 2005, 26, 359–371. [Google Scholar] [CrossRef] [PubMed]
- De la Riva, B.; Nowak, C.; Sánchez, E.; Hernández, A.; Schulz-Siegmund, M.; Pec, M.K.; Delgado, A.; Évora, C. VEGF-controlled release within a bone defect from alginate/chitosan/PLA-H scaffolds. Eur. J. Pharm. Biopharm. 2009, 73, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Köwitsch, A.; Ma, N.; Mäder, K.; Pashkuleva, I.; Reis, R.L.; Groth, T. Functionality of surface-coupled oxidised glycosaminoglycans towards fibroblast adhesion. J. Bioact. Compat. Polym. 2016, 31, 191–207. [Google Scholar] [CrossRef]
- Köwitsch, A.; Yang, Y.; Ma, N.; Kuntsche, J.; Mäder, K.; Groth, T. Bioactivity of immobilized hyaluronic acid derivatives regarding protein adsorption and cell adhesion. Biotechnol. Appl. Biochem. 2011, 58, 376–389. [Google Scholar] [CrossRef]
- Korzhikov, V.; Roeker, S.; Vlakh, E.; Kasper, C.; Tennikova, T. Synthesis of multifunctional polyvinylsaccharide containing controllable amounts of biospecific ligands. Bioconjugate Chem. 2008, 19, 617–625. [Google Scholar] [CrossRef]
- Zhao, M.; Li, L.; Zhou, C.; Heyroth, F.; Fuhrmann, B.; Maeder, K.; Groth, T. Improved stability and cell response by intrinsic cross-linking of multilayers from collagen I and oxidized glycosaminoglycans. Biomacromolecules 2014, 15, 4272–4280. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 10993-3, Third Edition: Biological Evaluation of Medical Devices—Part 3: Tests for Genotoxicity, Carcinogenicity and Reproductive Toxicity; International Organization for Standardization: Geneva, Switzerland, 2014. [Google Scholar]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA 2004, 101, 9607–9611. [Google Scholar] [CrossRef] [Green Version]
- Alarake, N.Z.; Frohberg, P.; Groth, T.; Pietzsch, M. Mechanical properties and biocompatibility of in situ enzymatically cross-linked gelatin hydrogels. Int. J. Artif. Organs 2017, 40, 159–168. [Google Scholar] [CrossRef]
- Morra, M. Engineering of biomaterials surfaces by hyaluronan. Biomacromolecules 2005, 6, 1205–1223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xue, M.; Yang, J.; Tan, T. A novel injectable and in situ crosslinked hydrogel based on hyaluronic acid and α, β-polyaspartylhydrazide. J. Appl. Polym. Sci. 2012, 125, 1116–1126. [Google Scholar] [CrossRef]
- Jejurikar, A.; Seow, X.T.; Lawrie, G.; Martin, D.; Jayakrishnan, A.; Grøndahl, L. Degradable alginate hydrogels crosslinked by the macromolecular crosslinker alginate dialdehyde. J. Mater. Chem. 2012, 22, 9751–9758. [Google Scholar] [CrossRef]
- Emami, Z.; Ehsani, M.; Zandi, M.; Foudazi, R. Controlling alginate oxidation conditions for making alginate-gelatin hydrogels. Carbohydr. Polym. 2018, 198, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Yegappan, R.; Selvaprithiviraj, V.; Mohandas, A.; Jayakumar, R. Nano polydopamine crosslinked thiol-functionalized hyaluronic acid hydrogel for angiogenic drug delivery. Colloids Surf. B Biointerfaces 2019, 177, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Bouhadir, K.H.; Lee, K.Y.; Alsberg, E.; Damm, K.L.; Anderson, K.W.; Mooney, D.J. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol. Prog. 2001, 17, 945–950. [Google Scholar] [CrossRef]
- Strätz, J.; Liedmann, A.; Heinze, T.; Fischer, S.; Groth, T. Effect of sulfation route and subsequent oxidation on derivatization degree and biocompatibility of cellulose sulfates. Macromol. Biosci. 2020, 20, 1900403. [Google Scholar] [CrossRef] [Green Version]
- Elahipanah, S.; O’Brien, P.J.; Rogozhnikov, D.; Yousaf, M.N. General dialdehyde click chemistry for amine bioconjugation. Bioconjugate Chem. 2017, 28, 1422–1433. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Qhattal, H.S.S.; Liu, X. Characterization of CD44-mediated cancer cell uptake and intracellular distribution of hyaluronan-grafted liposomes. Mol. Pharm. 2011, 8, 1233–1246. [Google Scholar] [CrossRef] [Green Version]
- Andersen, T.; Auk-Emblem, P.; Dornish, M. 3D cell culture in alginate hydrogels. Microarrays 2015, 4, 133–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.J.; Hinner, M.J. Getting across the cell membrane: An overview for small molecules, peptides, and proteins. In Site-Specific Protein Labeling; Springer: Berlin/Heidelberg, Germany, 2015; pp. 29–53. [Google Scholar]
- Poveda-Reyes, S.; Moulisova, V.; Sanmartín-Masiá, E.; Quintanilla-Sierra, L.; Salmerón-Sánchez, M.; Ferrer, G.G. Gelatin—Hyaluronic acid hydrogels with tuned stiffness to counterbalance cellular forces and promote cell differentiation. Macromol. Biosci. 2016, 16, 1311–1324. [Google Scholar] [CrossRef] [PubMed]
- Poveda-Reyes, S.; Rodrigo-Navarro, A.; Gamboa-Martínez, T.C.; Rodíguez-Cabello, J.C.; Quintanilla-Sierra, L.; Edlund, U.; Ferrer, G.G. Injectable composites of loose microfibers and gelatin with improved interfacial interaction for soft tissue engineering. Polymer 2015, 74, 224–234. [Google Scholar] [CrossRef]







| Amount of NaIO4 Used (eq) | Reaction Time (h) | DOexp (%) Titration | DSCHO Titration | DOexp (%) UV/VIS Spectro-Scopy | DSCHO UV/VIS Spectro-Scopy |
|---|---|---|---|---|---|
| 0.25 | 3 | 12.5 | 0.25 | 9 | 0.18 |
| 0.25 | 24 | 13 | 0.26 | 9 | 0.18 |
| 0.5 | 3 | 15.5 | 0.31 | 12.5 | 0.25 |
| 1 | 3 | 21.5 | 0.43 | 18 | 0.36 |
| 1.2 | 24 | 23.5 | 0.48 | 21 | 0.42 |
| 1.2 | 72 | 24.5 | 0.49 | 22.5 | 0.45 |
| Amount of NaIO4 Used (eq) | Reaction Time (h) | DOexp (%) Titration | DSCHO Titration | DOexp (%) UV/VIS Spectro-Scopy | DSCHO UV/VIS Spectro-Scopy |
|---|---|---|---|---|---|
| 0.5 | 6 | 1 | 0.02 | 1 | 0.02 |
| 0.5 | 24 | 6 | 0.12 | 3.5 | 0.07 |
| 1 | 6 | 4 | 0.08 | 2.5 | 0.05 |
| 1 | 24 | 25.5 | 0.51 | 22 | 0.44 |
| 1.2 | 24 | 25 | 0.5 | 21 | 0.42 |
| 1.2 | 72 | 36 | 0.72 | 36 | 0.72 |
| Hydrogel | G’ (Pa) | G” (Pa) | Gelation Time (min) |
|---|---|---|---|
| CMC-oALGDS 0.25 | 7 ± 4 | 5.2 ± 0.9 | >30 min |
| CMC-oALGDS 0.49 | 111 ± 29 | 8.4 ± 2.2 | 16 ± 4 |
| CMC-oHADS 0.02 | 11 ± 9 | 5.1 ± 2.7 | >30 min |
| CMC-oHADS 0.72 | 323 ± 16 | 11.7 ± 1.6 | 8 ± 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, M.; Willems, C.; Rodríguez-Fernández, J.; Gallego-Ferrer, G.; Groth, T. Synthesis and Characterization of Oxidized Polysaccharides for In Situ Forming Hydrogels. Biomolecules 2020, 10, 1185. https://doi.org/10.3390/biom10081185
Muhammad M, Willems C, Rodríguez-Fernández J, Gallego-Ferrer G, Groth T. Synthesis and Characterization of Oxidized Polysaccharides for In Situ Forming Hydrogels. Biomolecules. 2020; 10(8):1185. https://doi.org/10.3390/biom10081185
Chicago/Turabian StyleMuhammad, Muhammad, Christian Willems, Julio Rodríguez-Fernández, Gloria Gallego-Ferrer, and Thomas Groth. 2020. "Synthesis and Characterization of Oxidized Polysaccharides for In Situ Forming Hydrogels" Biomolecules 10, no. 8: 1185. https://doi.org/10.3390/biom10081185