Ginseng Gintonin Attenuates Lead-Induced Rat Cerebellar Impairments during Gestation and Lactation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animals
2.2. Preparation of Gintonin
2.3. Administration of Lead Acetate and Gintonin
2.4. Locomotor Coordination Assay (Bar Holding Test and Wire Mesh Ascending Test) in Offspring
2.5. Analysis of Pb Level in the Blood Using Atomic Absorption Spectrometry and Measurement of Cerebellar Weight
2.6. Tissue Processing, Nissl Staining, and Immunohistochemistry
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
3.1. Effects of Gintonin Administration on Pb-Induced Changes in Body Weight, Cerebellar Weight, and Blood Pb Levels
3.2. Gintonin Treatment Attenuates Pb-Induced Damages in the Developing Cerebellum
3.3. Gintonin Treatment Attenuates Pb-Induced Changes in BDNF, Sirt1, and Apoptosis-Related Bax and Bcl2 Protein Expression
3.4. Gintonin Treatment Attenuates Pb-Induced Changes in LPAR1, Presynaptic Synaptophysin, and Post-NMDAR1 Expression
3.5. Gintonin Treatment Attenuates Pb-Induced Changes in GAD and GABAT1
3.6. Effects of Gintonin Treatment on Pb-Induced Changes in Nrf2, Mn-SOD, IL-1β, and TNFα Expression
3.7. Effects of Gintonin Co-Administration with Pb on MBP, Olig2, and Locomotive Functions in the Developing Cerebellum
4. Discussion
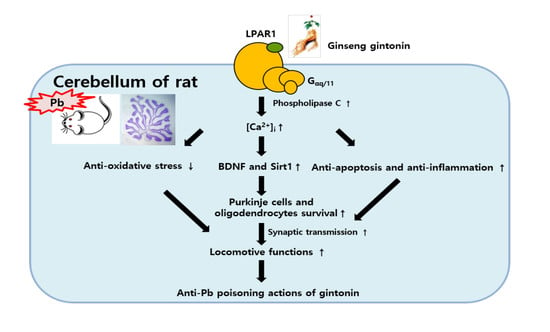
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, H.J.; Kim, D.J.; Shin, E.J.; Lee, B.H.; Choi, S.H.; Hwang, S.H.; Rhim, H.; Cho, I.H.; Kim, H.C.; Nah, S.Y. Effects of gintonin-enriched fraction on hippocampal cell proliferation in wild-type mice and an APPswe/PSEN-1 double Tg mouse model of Alzheimer’s disease. Neurochem. Int. 2016, 101, 56–65. [Google Scholar] [CrossRef]
- Cho, H.J.; Choi, S.H.; Kim, H.J.; Lee, B.H.; Rhim, H.; Kim, H.C.; Hwang, S.H.; Nah, S.Y. Bioactive lipids in gintonin-enriched fraction from ginseng. J. Ginseng Res. 2019, 43, 209–217. [Google Scholar] [CrossRef]
- Jang, M.; Choi, J.H.; Chang, Y.; Lee, S.J.; Nah, S.Y.; Cho, I.H. Gintonin, a ginseng-derived ingredient, as a novel therapeutic strategy for Huntington’s disease: Activation of the Nrf2 pathway through lysophosphatidic acid receptors. Brain Behav. Immu. 2019, 80, 146–162. [Google Scholar] [CrossRef]
- Jo, M.G.; Ikram, M.; Jo, M.H.; Yoo, L.; Chung, K.C.; Nah, S.Y.; Hwang, H.; Rhim, H.; Kim, M.O. Gintonin mitigates MPTP-induced loss of nigrostriatal dopaminergic neurons and accumulation of α-synuclein via the Nrf2/HO-1 pathway. Mol. Neurobiol. 2019, 56, 39–55. [Google Scholar] [CrossRef]
- Lee, M.J.; Chang, B.J.; Oh, S.; Nah, S.Y.; Cho, I.H. Korean red ginseng mitigates spinal demyelination in a model of acute multiple sclerosis by downregulating p38 mitogen-activated protein kinase and nuclear factor-κB signaling pathways. J. Ginseng Res. 2018, 42, 436–446. [Google Scholar] [CrossRef]
- Leung, K.W.; Wong, A.S.T. Pharmacology of ginsenosides: a literature review. Chin. Med. 2010, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.H.; Shin, T.J.; Choi, S.H.; Cho, H.J.; Lee, B.H.; Pyo, M.K.; Lee, J.H.; Kang, J.; Kim, H.J.; Park, C.W.; et al. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates G protein-coupled lysophosphatidic acid receptors with high affinity. Mol. Cells 2012, 33, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Jung, S.W.; Lee, B.H.; Kim, H.J.; Hwang, S.H.; Kim, H.K.; Nah, S.Y. Ginseng pharmacology: a new paradigm based on gintonin-lysophosphatidic acid receptor interactions. Front. Pharmacol. 2015, 6, 245. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.M.; Hwang, H.; Seo, M.; Chang, B.J.; Kim, H.J.; Choi, S.H.; Rhim, H.; Kim, H.C.; Cho, I.H.; Nah, S.Y. Gintonin attenuates D-galactose-induced hippocampal senescence by improving long-term hippocampal potentiation, neurogenesis, and cognitive functions. Gerontology 2018, 64, 562–575. [Google Scholar] [CrossRef]
- Rice, D.; Barone, S., Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 2000, 108, 511–533. [Google Scholar]
- Aghajanian, G.K.; Bloom, F.E. The formation of synaptic junctions in developing rat brain: a quantitative electron microscopic study. Brain Res. 1967, 6, 716–727. [Google Scholar] [CrossRef]
- Ye, X.; Fukushima, N.; Kingsbury, M.A.; Chun, J. Lysophosphatidic acid in neural signaling. Neuroreport 2002, 13, 2169–2175. [Google Scholar] [CrossRef]
- Castilla-Ortega, E.; Pedraza, C.; Chun, J.; de Fonseca, F.R.; Estivill-Torrús, G.; Santín, L.J. Hippocampal c-Fos activation in normal and LPA1-null mice after two object recognition tasks with different memory demands. Behav. Brain Res. 2012, 232, 400–405. [Google Scholar] [CrossRef]
- Suckau, O.; Gross, I.; Schrötter, S.; Yang, F.; Luo, J.; Wree, A.; Chun, J.; Baska, D.; Baumgart, J.; Kano, K.; et al. LPA1, LPA2, LPA4, and LPA6 receptor expression during mouse brain development. Dev. Dyn. 2019, 248, 375–395. [Google Scholar] [CrossRef] [Green Version]
- Parolisi, R.; Peruffo, A.; Messina, S.; Panin, M.; Montelli, S.; Giurisato, M.; Cozzi, B.; Bonfanti, L. Forebrain neuroanatomy of the neonatal and juvenile dolphin (T. truncatus and S. coeruloalba). Front. Neuroanat. 2015, 9, 140. [Google Scholar] [CrossRef] [Green Version]
- Kougias, D.G.; Cortes, L.R.; Moody, L.; Rhoads, S.; Pan, Y.X.; Juraska, J.M. Effects of perinatal exposure to phthalates and a high-fat diet on maternal behavior and pup development and social play. Endocrinology 2018, 159, 1088–1105. [Google Scholar] [CrossRef] [Green Version]
- Lanphear, B.P. The impact of toxins on the developing brain. Annu. Rev. Public Health 2015, 36, 211–230. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.M.; Cho, I.S.; Seo, J.S.; Go, T.H.; Kim, J.H.; Nahm, S.S.; Chang, B.J.; Lee, J.H. Ascorbic acid attenuates lead-induced alterations in the synapses in the developing rat cerebellum. Biol. Trace Elem. Res. 2019, 187, 142–150. [Google Scholar] [CrossRef]
- Tanaka, T.; Abe, H.; Kimura, M.; Onda, N.; Mizukami, S.; Yoshida, T.; Shibutani, M. Developmental exposure to T-2 toxin reversibly affects postnatal hippocampal neurogenesis and reduces neural stem cells and progenitor cells in mice. Arch. Toxicol. 2015, 90, 2009–2024. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Childhood lead poisoning. 2010. Available online: http://www.who.int/ceh/publications/childhoodpoisoning/en/ (accessed on 15 December 2019).
- United States Geological Survey (USGS). Lead. Mineral Commodities Summaries; National Minerals Information Center: Reston, VA, USA, 2017.
- Eom, S.Y.; Lee, Y.S.; Lee, S.G.; Seo, M.N.; Choi, B.S.; Kim, Y.D.; Lim, J.A.; Hwang, M.S.; Kwon, H.J.; Kim, Y.M.; et al. Lead, mercury, and cadmium exposure in the Korean general population. J. Korean Med. Sci. 2018, 33, e9. [Google Scholar] [CrossRef]
- Barkur, R.R.; Bairy, L.K. Histological study on hippocampus, amygdala and cerebellum following low lead exposure during prenatal and postnatal brain development in rats. Toxicol. Ind. Health. 2016, 32, 1052–1063. [Google Scholar] [CrossRef]
- Choi, S.H.; Jung, S.W.; Kim, H.S.; Kim, H.J.; Lee, B.H.; Kim, J.Y.; Kim, J.H.; Hwang, S.H.; Rhim, H.; Kim, H.C.; et al. A brief method for preparation of gintonin-enriched fraction from ginseng. J. Ginseng Res. 2015, 39, 398–405. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.M.; Seo, J.S.; Go, T.H.; Nahm, S.S.; Chang, B.J. Ascorbic acid supplementation prevents the detrimental effects of prenatal and postnatal lead exposure on the Purkinje cell and related proteins in the cerebellum of developing rats. Biol. Trace Elem. Res. 2019, 190, 446–456. [Google Scholar] [CrossRef]
- Nam, S.M.; Seo, J.S.; Nahm, S.S.; Chang, B.J. Effects of ascorbic acid on osteopontin expression and axonal myelination in the developing cerebellum of lead-exposed rat pups. Int. J. Environ. Res. Public Health 2019, 16, 983. [Google Scholar] [CrossRef] [Green Version]
- Takayama, C.; Inoue, Y. Developmental expression of GABA transporter-1 and 3 during formation of the GABAergic synapses in the mouse cerebellar cortex. Brain Res. Dev. Brain Res. 2005, 158, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.M.; Seo, J.S.; Nahm, S.S.; Chang, B.J. Effects of ascorbic acid treatment on developmental alterations in calcium-binding proteins and gamma-aminobutyric acid transporter 1 in the cerebellum of lead-exposed rats during pregnancy and lactation. J. Toxicol. Sci. 2019, 44, 799–809. [Google Scholar] [CrossRef]
- Nam, S.M.; Seo, M.; Seo, J.S.; Rhim, H.; Nahm, S.S.; Cho, I.H.; Chang, B.J.; Kim, H.J.; Choi, S.H.; Nah, S.Y. Ascorbic acid mitigates D-galactose-induced brain aging by increasing hippocampal neurogenesis and improving memory function. Nutrients 2019, 11, 176. [Google Scholar] [CrossRef] [Green Version]
- Whitney, E.R.; Kemper, T.L.; Rosene, D.L.; Bauman, M.L.; Blatt, G.J. Calbindin-D28k is a more reliable marker of human Purkinje cells than standard Nissl stains: a stereological experiment. J. Neurosci. Methods 2008, 168, 42–47. [Google Scholar] [CrossRef]
- Shen, J.; Xu, L.; Qu, C.; Sun, H.; Zhang, J. Resveratrol prevents cognitive deficits induced by chronic unpredictable mild stress: Sirt1/miR-134 signalling pathway regulates CREB/BDNF expression in hippocampus in vivo and in vitro. Behav. Brain Res. 2018, 349, 1–7. [Google Scholar] [CrossRef]
- Estivill-Torrús, G.; Llebrez-Zayas, P.; Matas-Rico, E.; Santín, L.; Pedraza, C.; De Diego, I.; Del Arco, I.; Fernández-Llebrez, P.; Chun, J.; De Fonseca, F.R. Absence of LPA1 signaling results in defective cortical development. Cereb. Cortex 2008, 18, 938–950. [Google Scholar] [CrossRef]
- Lidsky, T.I.; Schneider, J.S. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain 2003, 126, 5–19. [Google Scholar] [CrossRef]
- Wu, C.; Sun, D. GABA receptors in brain development, function, and injury. Metab. Brain Dis. 2015, 30, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Collin, L.; Usiello, A.; Erbs, E.; Mathis, C.; Borrelli, E. Motor training compensates for cerebellar dysfunctions caused by oligodendrocyte ablation. Proc. Natl. Acad. Sci. USA 2004, 101, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.H.; Jang, M.; Oh, S.; Nah, S.Y.; Cho, I.H. Multi-target protective effects of gintonin in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-mediated model of Parkinson’s disease via lysophosphatidic acid receptors. Front. Pharmacol. 2018, 9, 515. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, O.M.; Al Badawi, M.H.; Salem, N.A. Role of Ginseng on mercury chloride-induced testicular lesions in adult albino rat: a histological and immunohistochemical study. EJH 2014, 37, 506–513. [Google Scholar] [CrossRef]
- Shukla, R.; Kumar, M. Role of Panax ginseng as an antioxidant after cadmium-induced hepatic injuries. Food Chem. Toxicol. 2009, 47, 769–773. [Google Scholar] [CrossRef]
- Wang, B.; Feng, G.; Tang, C.; Wang, L.; Cheng, H.; Zhang, Y.; Ma, J.; Shi, M.; Zhao, G. Ginsenoside Rd maintains adult neural stem cell proliferation during lead-impaired neurogenesis. Neurol. Sci. 2013, 34, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Chang, B.J.; Li, T.Z.; Choe, N.H.; Quan, F.S.; Jang, B.J.; Cho, I.H.; Hong, H.N.; Lee, J.H. Protective effects of ascorbic acid against lead-induced apoptotic neurodegeneration in the developing rat hippocampus in vivo. Brain Res. 2007, 1185, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.M.; Chang, B.J.; Kim, J.H.; Nahm, S.S.; Lee, J.H. Ascorbic acid ameliorates lead-induced apoptosis in the cerebellar cortex of developing rats. Brain Res. 2018, 1686, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhou, C.C.; Yang, Y.; Liu, J.W.; Yan, C.H. GM1 ameliorates lead-induced cognitive deficits and brain damage through activating the SIRT1/CREB/BDNF pathway in the developing male rat hippocampus. Biol. Trace Elem. Res. 2019, 190, 425–436. [Google Scholar] [CrossRef]
- Linker, R.A.; Lee, D.H.; Demir, S.; Wiese, S.; Kruse, N.; Siglienti, I.; Gerhardt, E.; Neumann, H.; Sendtner, M.; Lühder, F.; et al. Functional role of brain-derived neurotrophic factor in neuroprotective autoimmunity: therapeutic implications in a model of multiple sclerosis. Brain 2010, 133, 2248–2263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, A.R.; Chen, C.; Schwartz, P.M.; Segal, R.A. Brain-derived neurotrophic factor modulates cerebellar plasticity and synaptic ultrastructure. J. Neurosci. 2002, 22, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Wu, X.; Cai, Q.; Wang, Y.; Xiao, L.; Tian, Y.; Li, H. Lead poisoning disturbs oligodendrocytes differentiation involved in decreased expression of NCX3 inducing intracellular calcium overload. Int. J. Mol. Sci. 2015, 16, 19096–19110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, P.M.; Borghesani, P.R.; Levy, R.L.; Pomeroy, S.L.; Segal, R.A. Abnormal cerebellar development and foliation in BDNF-/- mice reveals a role for neurotrophins in CNS patterning. Neuron 1997, 19, 269–281. [Google Scholar] [CrossRef] [Green Version]
- Hossain, S.; Bhowmick, S.; Jahan, S.; Rozario, L.; Sarkar, M.; Islam, S.; Basunia, M.A.; Rahman, A.; Choudhury, B.K.; Shahjalal, H. Maternal lead exposure decreases the levels of brain development and cognition-related proteins with concomitant upsurges of oxidative stress, inflammatory response and apoptosis in the offspring rats. Neurotoxicology 2016, 56, 150–158. [Google Scholar] [CrossRef]
- Toews, A.D.; Krigman, M.R.; Thomas, D.J.; Morell, P. Effect of inorganic lead exposure on myelination in the rat. Neurochem. Res. 1980, 5, 605–616. [Google Scholar] [CrossRef]
- Laursen, L.S.; Chan, C.W.; Ffrench-Constant, C. Translation of myelin basic protein mRNA in oligodendrocytes is regulated by integrin activation and hnRNP-K. J. Cell Biol. 2011, 192, 797–811. [Google Scholar] [CrossRef] [Green Version]
- Sheng, X.; Yung, Y.C.; Chen, A.; Chun, J. Lysophosphatidic acid signalling in development. Development 2015, 142, 1390–1395. [Google Scholar] [CrossRef] [Green Version]
- Hsu, P.C.; Liu, M.Y.; Hsu, C.C.; Chen, L.Y.; Guo, Y.L. Lead exposure causes generation of reactive oxygen species and functional impairment in rat sperm. Toxicology 1997, 122, 133–143. [Google Scholar] [CrossRef]
- Kraft, A.D.; Johnson, D.A.; Johnson, J.A. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J. Neurosci. 2004, 24, 1101–1112. [Google Scholar] [CrossRef] [Green Version]
- Metryka, E.; Chibowska, K.; Gutowska, I.; Falkowska, A.; Kupnicka, P.; Barczak, K.; Chlubek, D.; Baranowska-Bosiacka, I. Lead (Pb) exposure enhances expression of factors associated with inflammation. Int. J. Mol. Sci. 2018, 19, 1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, S.M.; Choi, S.-H.; Cho, H.-J.; Seo, J.S.; Choi, M.; Nahm, S.-S.; Chang, B.-J.; Nah, S.-Y. Ginseng Gintonin Attenuates Lead-Induced Rat Cerebellar Impairments during Gestation and Lactation. Biomolecules 2020, 10, 385. https://doi.org/10.3390/biom10030385
Nam SM, Choi S-H, Cho H-J, Seo JS, Choi M, Nahm S-S, Chang B-J, Nah S-Y. Ginseng Gintonin Attenuates Lead-Induced Rat Cerebellar Impairments during Gestation and Lactation. Biomolecules. 2020; 10(3):385. https://doi.org/10.3390/biom10030385
Chicago/Turabian StyleNam, Sung Min, Sun-Hye Choi, Hee-Jung Cho, Jin Seok Seo, Minsuk Choi, Sang-Soep Nahm, Byung-Joon Chang, and Seung-Yeol Nah. 2020. "Ginseng Gintonin Attenuates Lead-Induced Rat Cerebellar Impairments during Gestation and Lactation" Biomolecules 10, no. 3: 385. https://doi.org/10.3390/biom10030385