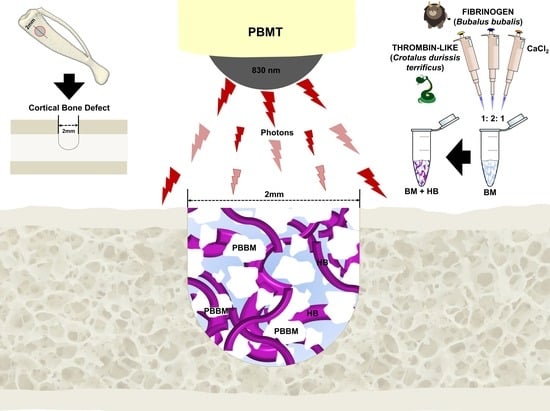
Photobiomodulation Therapy Associated with Heterologous Fibrin Biopolymer and Bovine Bone Matrix Helps to Reconstruct Long Bones
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Maintenance
2.2. Experimental Design
2.3. Lyophilized Bovine Bone Matrix (BM)
2.4. Heterologous Fibrin Biopolymer (HFB)
2.5. Monocortical Defect Surgery
2.6. Photobiomodulation Therapy
2.7. Collection of Samples and Histological Procedures
2.8. X-ray Computed Microtomography Analysis (µ-CT)
2.9. Histomorphometric and Histological Analysis
2.10. Statistical Analysis
3. Results
3.1. General Evaluation
3.2. Microtomographic Evaluation
3.3. Histological Evaluation
3.4. Histomorphometric Evaluation
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaur, G.; Grover, V.; Bhaskar, N.; Kaur, R.K.; Jain, A. Periodontal Infectogenomics. Inflamm. Regen. 2018, 38, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, D.; Zhang, Y.; Li, M. Inflammation, mesenchymal stem cells and bone regeneration. Histochem. Cell Biol. 2018, 16, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater Res. 2019, 23, 4–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasouli Ghahroudi, A.; Rokn, A.; Kalhori, K.; Khorsand, A.; Pournabi, A.; Pinheiro, A.; Fekrazad, R. Effect of low-level laser therapy irradiation and Bio-Oss® graft material on the osteogenesis process in rabbit calvarium defects: A double blind experimental study. Lasers Med Sci. 2014, 29, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.B.; Kim, H.J.; Ahn, J.J.; Bae, H.Y.; Kim, H.J.; Huh, J.B. Comparison of bone regeneration between porcine-derived and bovine-derived xenografts in rat calvarial defects: A non-inferiority study. Materials 2019, 12, 3412. [Google Scholar] [CrossRef] [Green Version]
- Biscola, N.P.; Cartarozzi, L.P.; Ulian-Benitez, S.; Barbizan, R.; Castro, M.V.; Spejo, A.B.; Ferreira, R.S.; Barraviera, B.; Oliveira, A.L.R. Multiple uses of fibrin sealant for nervous system treatment following injury and disease. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 13. [Google Scholar] [CrossRef] [Green Version]
- Carmagnola, D.; Berglundh, T.; Araujo, M.; Albrektsson, T.; Lindhe, J. Bone healing around implants placed in a jaw defect augmented with Bio-Oss®. An experimental study in dogs. J. Clin. Periodontol. 2000, 27, 799–805. [Google Scholar] [CrossRef]
- Pomini, K.T.; Buchaim, D.V.; Andreo, J.C.; Rosso, M.P.O.; Della Coletta, B.B.; German, Í.J.S.; Biguetti, A.C.C.; Shinohara, A.L.; Rosa Júnior, G.M.; Cosin Shindo, J.V.T.; et al. Fibrin Sealant Derived from Human Plasma as a Scaffold for Bone Grafts Associated with Photobiomodulation Therapy. Int. J. Mol. Sci. 2019, 20, 1761. [Google Scholar] [CrossRef] [Green Version]
- Buchaim, D.V.; Cassaro, C.V.; Shindo, J.V.T.C.; Coletta, B.B.D.; Pomini, K.T.; Rosso, M.P.O.; Campos, L.M.G.; Ferreira, R.S., Jr.; Barraviera, B.; Buchaim, R.L. Unique hetetologous fibrin biopolymer with hemostatic, adhesive, sealant, scaffold and drug delivery properties—A systematic review. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25. [Google Scholar] [CrossRef]
- Ferreira, R.S.; de Barros, L.C.; Abbade, L.P.F.; Barraviera, S.R.C.S.; Silvares, M.R.C.; de Pontes, L.G.; dos Santos, L.D.; Barraviera, B. Heterologous fibrin sealant derived from snake venom: From bench to bedside—An overview. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 21. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, R.S. Autologous or heterologous fibrin sealant scaffold: Which is the better choice? J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchaim, D.V.; Rodrigues, A.D.C.; Buchaim, R.L.; Barraviera, B.; Junior, R.S.F.; Junior, G.M.R.; Bueno, C.R.D.S.; Roque, D.D.; Dias, D.V.; Dare, L.R.; et al. The new heterologous fibrin sealant in combination with low-level laser therapy (LLLT) in the repair of the buccal branch of the facial nerve. Lasers Med. Sci. 2016, 31, 965–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosso, M.P.D.O.; Rosa Júnior, G.M.; Buchaim, D.V.; German, I.J.S.; Pomini, K.T.; de Souza, R.G.; Pereira, M.; Favaretto Júnior, I.A.; Bueno, C.R.D.S.; Gonçalves, J.B.D.O.; et al. Stimulation of morphofunctional repair of the facial nerve with photobiomodulation, using the end-to-side technique or a new heterologous fibrin sealant. J. Photochem. Photobiol. B Biol. 2017, 175, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orsi, P.R.; Landim-Alvarenga, F.C.; Justulin, L.A.; Kaneno, R.; De Assis Golim, M.; Dos Santos, D.C.; Creste, C.F.Z.; Oba, E.; Maia, L.; Barraviera, B.; et al. A unique heterologous fibrin sealant (HFS) as a candidate biological scaffold for mesenchymal stem cells in osteoporotic rats. Stem Cell Res. Ther. 2017, 8, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassaro, C.; Justulin, L., Jr.; Lima, P.; Golim, M.; Biscola, N.; Castro, M.; Oliveira, A.; Doiche, D.; Pereira, E.; Ferreira, R., Jr.; et al. Fibrin biopolymer as scaffold candidate to treat bone defects in rats. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira Gonçalves, J.B.; Buchaim, D.V.; de Souza Bueno, C.R.; Pomini, K.T.; Barraviera, B.; Júnior, R.S.F.; Andreo, J.C.; de Castro Rodrigues, A.; Cestari, T.M.; Buchaim, R.L. Effects of low-level laser therapy on autogenous bone graft stabilized with a new heterologous fibrin sealant. J. Photochem. Photobiol. B Biol. 2016, 162, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Iatecola, A.; Barraviera, B.; Junior, R.S.F.; dos Santos, G.R.; Neves, J.I.; da Cunha, M.R. Use of a new fibrin sealant and laser irradiation in the repair of skull defects in rats. Braz. Dent. J. 2013, 24, 456–461. [Google Scholar] [CrossRef]
- Tim, C.R.; Bossini, P.S.; Kido, H.W.; Malavazi, I.; Von Zeska Kress, M.R.; Carazzolle, M.F.; Parizotto, N.A.; Rennó, A.C. Effects of low level laser therapy on inflammatory and angiogenic gene expression during the process of bone healing: A microarray analysis. J. Photochem. Photobiol. B Biol. 2016, 154, 8–15. [Google Scholar] [CrossRef]
- Zein, R.; Selting, W.; Hamblin, M.R. Review of light parameters and photobiomodulation efficacy: Dive into complexity. J. Biomed. Opt. 2018, 23, 120901. [Google Scholar] [CrossRef] [Green Version]
- Karu, T.; Pyatibrat, L.; Kalendo, G. Irradiation with He-Ne laser increases ATP level in cells cultivated in vitro. J. Photochem. Photobiol. B 1995, 27, 219–223. [Google Scholar] [CrossRef]
- De Freitas, L.; Hamblin, M. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tani, A.; Chellini, F.; Giannelli, M.; Nosi, D.; Zecchi-Orlandini, S.; Sassoli, C. Red (635 nm), near-infrared (808 nm) and violet-blue (405 nm) photobiomodulation potentiality on human osteoblasts and mesenchymal stromal cells: A morphological and molecular in vitro study. Int. J. Mol. Sci. 2018, 19, 1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosso, M.P.O.; Buchaim, D.V.; Pomini, K.T.; Coletta, B.B.D.; Reis, C.H.B.; Pilon, J.P.G.; Duarte Júnior, G.; Buchaim, R.L. Photobiomodulation Therapy (PBMT) Applied in Bone Reconstructive Surgery Using Bovine Bone Grafts: A Systematic Review. Materials 2019, 12, 4051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosso, M.P.O.; Buchaim, D.V.; Kawano, N.; Furlanette, G.; Pomini, K.T.; Buchaim, R.L. Photobiomodulation Therapy (PBMT) in Peripheral Nerve Regeneration: A Systematic Review. Bioengineering 2018, 5, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosso, M.P.O.; Buchaim, D.V.; Rosa Junior, G.M.; Andreo, J.C.; Pomini, K.T.; Buchaim, R.L. Low-Level Laser Therapy (LLLT) Improves the Repair Process of Peripheral Nerve Injuries: A Mini Review. Int. J. Neurorehabilitation 2017, 4, 2–4. [Google Scholar]
- Pekkan, G.; Aktas, A.; Pekkan, K. Comparative radiopacity of bone graft materials. J. Cranio-Maxillofac. Surg. 2012, 40, e1–e4. [Google Scholar] [CrossRef]
- Jensen, T.; Schou, S.; Stavropoulos, A.; Terheyden, H.; Holmstrup, P. Maxillary sinus floor augmentation with Bio-Oss or Bio-Oss mixed with autogenous bone as graft: A systematic review. Clin. Oral Implants Res. 2012, 23, 263–273. [Google Scholar] [CrossRef]
- De Santis, E.; Lang, N.P.; Ferreira, S.; Rangel Garcia, I.; Caneva, M.; Botticelli, D. Healing at implants installed concurrently to maxillary sinus floor elevation with Bio-Oss® or autologous bone grafts. A histo-morphometric study in rabbits. Clin. Oral Implants Res. 2017, 28, 503–511. [Google Scholar] [CrossRef]
- Carinci, F.; Piattelli, A.; Degidi, M.; Palmieri, A.; Perrotti, V.; Scapoli, L.; Martinelli, M.; Laino, G.; Pezzetti, F. Genetic effects of anorganic bovine bone (Bio-Oss®) on osteoblast-like MG63 cells. Arch. Oral Biol. 2006, 51, 154–163. [Google Scholar] [CrossRef]
- Gasparotto, V.P.; Landim-Alvarenga, F.C.; Oliveira, A.L.; Simões, G.F.; Lima-Neto, J.F.; Barraviera, B.; Ferreira, R.S. A new fibrin sealant as a three-dimensional scaffold candidate for mesenchymal stem cells. Stem Cell Res. Ther. 2014, 5, 78. [Google Scholar] [CrossRef] [Green Version]
- Fangel, R.; Bossini, P.S.; Renno, A.C.; Granito, R.N.; Wang, C.C.; Nonaka, K.O.; Driusso, P.; Parizotto, N.A.; Oishi, J. Biomechanical properties: Effects of low-level laser therapy and Biosilicate® on tibial bone defects in osteopenic rats. J. Appl. Biomater. Funct. Mater. 2014, 12, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Kido, H.W.; Bossini, P.S.; Tim, C.R.; Parizotto, N.A.; da Cunha, A.F.; Malavazi, I.; Renno, A.C.M. Evaluation of the bone healing process in an experimental tibial bone defect model in ovariectomized rats. Aging Clin. Exp. Res. 2014, 26, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Bossini, P.S.; Rennó, A.C.M.; Ribeiro, D.A.; Fangel, R.; Ribeiro, A.C.; de Assis Lahoz, M.; Parizotto, N.A. Low level laser therapy (830nm) improves bone repair in osteoporotic rats: Similar outcomes at two different dosages. Exp. Gerontol. 2012, 47, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Prado, F.A.; Anbinder, A.L.; Jaime, A.P.G.; Lima, A.P.; Balducci, I.; Rocha, R.F. Bone defect in rat tibia: Standardization of experimental model. Rev. Odontol. UNICID 2006, 18, 7–13. [Google Scholar]
- Li, J.; Wu, H.; Zhang, S.; Jiang, H.; Wang, H.; Gao, Y.; Cheng, P.; Pan, D.; Li, D.; Yang, L.; et al. MIF Promotes Bone Defect Repair by Regulating Macrophages. Cell Stem Cells Regen. Med. 2018, 3, 2–6. [Google Scholar]
- Kido, H.; Brassolatti, P.; Tim, C.; Gabbai-Armelin, P.; Magri, A.P.; Fernandes, K.; Bossini, P.; Parizotto, N.; Crovace, M.; Malavazi, I.; et al. Porous poly (D,L-lactide-co-glycolide) acid/biosilicate® composite scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 63–71. [Google Scholar] [CrossRef]
- Garavello-Freitas, I.; Baranauskas, V.; Joazeiro, P.P.; Padovani, C.R.; Dal Pai-Silva, M.; da Cruz-Höfling, M.A. Low-power laser irradiation improves histomorphometrical parameters and bone matrix organization during tibia wound healing in rats. J. Photochem. Photobiol. B Biol. 2003, 70, 81–89. [Google Scholar] [CrossRef]
- Buchaim, R.L.; Rosso, M.P.O.; Andreo, J.C.; Buchaim, D.V.; Okamoto, R.; Rodrigues, A.C.; Shinohara, A.L.; Roque, J.S.; Roque, D.D.; Junior, G.M.R.; et al. A New Anionic Bovine Tendon as Scaffold for the Repair of Bone Defects: A Morphological, Histomorphometric and Immunohistochemical Study. Br. J. Med. Med. Res. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Buchaim, D.V.; Bueno, P.C.D.S.; Andreo, J.C.; Roque, D.D.; Roque, J.S.; Zilio, M.G.; Salatin, J.A.; Kawano, N.; Furlanette, G.; Buchaim, R.L. Action of a deproteinized xenogenic biomaterial in the process of bone repair in rats submitted to inhalation of cigarette smoke. Acta Cir. Bras. 2018, 33, 324–332. [Google Scholar] [CrossRef] [Green Version]
- Mandarim-de-lacerda, C.A. Stereological tools in biomedical research. An. Acad. Bras. Cienc. 2003, 75, 469–486. [Google Scholar] [CrossRef]
- Soares, L.G.; Marques, A.M.; Aciole, J.M.; da Guarda, M.G.; Cangussú, M.C.; Silveira, L., Jr.; Pinheiro, A.L. Do laser/LED phototherapies influence the outcome of the repair of surgical bone defects grafted with biphasic synthetic microgranular HA + β-tricalcium phosphate? A Raman spectroscopy study. Lasers Med. Sci. 2014, 29, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.; Griffin, M.; Hutmacher, D. Animal models for bone tissue engineering and modelling disease. Dis. Model. Mech. 2018, 11, dmm033084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Yeung, K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Lee, J.; Yun, H.; Shin, H.; Park, E. Comparison of bone regeneration rate in flat and long bone defects: Calvarial and tibial bone. Tissue Eng. Regen. Med. 2013, 10, 336–340. [Google Scholar] [CrossRef]
- dos Santos, P.; de Molon, R.; Queiroz, T.; Okamoto, R.; de Souza Faloni, A.; Gulinelli, J.; Luvizuto, E.; Garcia, I. Evaluation of bone substitutes for treatment of peri-implant bone defects: Biomechanical, histological, and immunohistochemical analyses in the rabbit tibia. J. Periodontal Implant Sci. 2016, 46, 176–196. [Google Scholar] [CrossRef] [Green Version]
- Buchaim, R.L.; Andreo, J.C.; Rodrigues, A.D.C.; Buchaim, D.V.; Roque, D.D.; Roque, J.S.; Rosa Júnior, G.M. Bovine Bone Matrix Action Associated With Morphogenetic Protein in Bone Defects in Rats Submitted to Alcoholism. Int. J. Morphol. 2012, 30, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Buchaim, R.L.; Goissis, G.; Andreo, J.C.; Roque, D.D.; Roque, J.S.; Buchaim, D.V.; Rodrigues, A.C. Biocompatibility of anionic collagen matrices and its influence on the orientation of cellular growth. Braz. Dent. Sci. 2007, 10, 12–20. [Google Scholar]
- Buchaim, R.L.; Roque, D.D.; Roque, J.S.; Toledo Filho, J.L.; Andreo, J.C.; Okamoto, T. Gen-phos® implant in surgical cavities performed in the tibia of rats submitted to experimental chronic alcoholism. A microscopic study. Rev. FOB 2002, 10, 17–22. [Google Scholar]
- Song, S.H.; Yun, Y.P.; Kim, H.J.; Park, K.; Kim, S.E.; Song, H.R. Bone Formation in a Rat Tibial Defect Model Using Carboxymethyl Cellulose / BioC / Bone Morphogenic Protein-2 Hybrid Materials. Biomed. Res. Int. 2014, 2014, 230152. [Google Scholar] [CrossRef]
- Cunha, M.J.; Esper, L.A.; Sbrana, M.C.; Oliveira, P.G.; Valle, A.L.; Almeida, A. Effect of Low-Level Laser on Bone Defects Treated with Bovine or Autogenous Bone Grafts: In Vivo Study in Rat Calvaria. Biomed. Res. Int. 2014, 2014, 104230. [Google Scholar] [CrossRef]
- Havlucu, U.; Bölükbaşı, N.; Yeniyol, S.; Çetin, Ş.; Özdemir, T. Effects of Light-Emitting Diode Photobiomodulation Therapy and BioOss as Single and Combined Treatment in an Experimental Model of Bone Defect Healing in Rats. J. Oral Implantol. 2014, 41, e110–e117. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.M.; Doering, H.; Schmidt, T.; Lutz, R.; Neukam, F.W.; Schlegel, K.A. Histological results after maxillary sinus augmentation with Straumann® BoneCeramic, Bio-Oss®, Puros®, and autologous bone. A randomized controlled clinical trial. Clin. Oral Implants Res. 2013, 24, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Kapogianni, E.; Barbeck, M.; Jung, O.; Arslan, A.; Kuhnel, L.; Xiong, X.; Krastev, R.; Friedrich, R.; Schnettler, R.; Fienitz, T.; et al. Comparison of Material-mediated Bone Regeneration Capacities of Sintered and Non-sintered Xenogeneic Bone Substitutes via 2D and 3D Data. In Vivo 2019, 33, 2169–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnet, N.; Laroche, N.; Vico, L.; Dolleans, E.; Courteix, D.; Benhamou, C.L. Assessment of trabecular bone microarchitecture by two different x-ray microcomputed tomographs: A comparative study of the rat distal tibia using Skyscan and Scanco devices. Med. Phys. 2009, 36, 1286–1297. [Google Scholar] [CrossRef]
- Gatti, M.A.N.; Vieira, L.M.; Barraviera, B.; Barraviera, S.R.C.S. Treatment of venous ulcers with fibrin sealant derived from snake venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2011, 17, 226–229. [Google Scholar] [CrossRef]
- Barros, L.C.; Ferreira, R.S.; Barraviera, S.R.C.S.; Stolf, H.O.; Thomazini-Santos, I.A.; Mendes-Giannini, M.J.S.; Toscano, E.; Barraviera, B. A new fibrin sealant from crotalus durissus terrificus venom: Applications in medicine. J. Toxicol. Environ. Health Part B Crit. Rev. 2009, 12, 553–571. [Google Scholar] [CrossRef]
- Buchaim, R.L.; Andreo, J.C.; Barraviera, B.; Ferreira Junior, R.S.; Buchaim, D.V.; Rosa Junior, G.M.; De Oliveira, A.L.R.; De Castro Rodrigues, A. Effect of low-level laser therapy (LLLT) on peripheral nerve regeneration using fibrin glue derived from snake venom. Injury 2015, 46, 655–660. [Google Scholar] [CrossRef]
- Machado, E.G.; Issa, J.P.M.; De Figueiredo, F.A.T.; Dos Santos, G.R.; Galdeano, E.A.; Alves, M.C.; Chacon, E.L.; Ferreira Junior, R.S.; Barraviera, B.; da Cunha, M.R. A new heterologous fibrin sealant as scaffold to recombinant human bone morphogenetic protein-2 (rhBMP-2) and natural latex proteins for the repair of tibial bone defects. Acta Histochem. 2015, 117, 288–296. [Google Scholar] [CrossRef]
- De Oliveira, P.; Fernandes, K.R.; Sperandio, E.F.; Pastor, F.A.C.; Nonaka, K.O.; Parizotto, N.A.; Renno, A.C.M. Comparative study of the effects of low-level laser and low-intensity ultrasound associated with Biosilicate® on the process of bone repair in the rat tibia. Rev. Bras. Ortop. 2012, 47, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Paolillo, A. Assessment of Bone Repair by X-ray Microtomography in Complete Tibia Rats osteotomy after Low-Intensity Pulsed Ultrasound and Low-Level Laser Therapy. Ph.D. Thesis, University of São Paulo, São Carlos, Brazil, 2013. [Google Scholar]
- Fávaro-Pípi, E.; Feitosa, S.M.; Ribeiro, D.A.; Bossini, P.; Oliveira, P.; Parizotto, N.A.; Renno, A.C.M. Comparative study of the effects of low-intensity pulsed ultrasound and low-level laser therapy on bone defects in tibias of rats. Lasers Med. Sci. 2010, 25, 727–732. [Google Scholar] [CrossRef]
- Fernandes, K.R.; Magri, A.M.P.; Kido, H.W.; Parisi, J.R.; Assis, L.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; Martins, V.C.; Plepis, A.M.; Zanotto, E.D.; et al. Biosilicate/PLGA osteogenic effects modulated by laser therapy: In vitro and in vivo studies. J. Photochem. Photobiol. B Biol. 2017, 173, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Gerbi, M.E.M.; Marques, A.M.C.; Ramalho, L.M.P.; Ponzi, E.A.C.; Carvalho, C.M.; Santos, R.D.C.; Oliveira, P.C.; Nóia, M.; Pinheiro, A.L.B. Infrared Laser Light Further Improves Bone Healing When Associated with Bone Morphogenic Proteins: An in Vivo Study in a Rodent Model. Photomed. Laser Surg. 2008, 26, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Márquez Martínez, M.; Pinheiro, A.; Ramalho, L. Effect of IR laser photobiomodulation on the repair of bone defects grafted with organic bovine bone. Lasers Med. Sci. 2008, 23, 313–317. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, G.; Aroni, M.; Medeiros, M.; Marcantonio, E.; Marcantonio, R. Effect of low-level laser therapy on the healing of sites grafted with coagulum, deproteinized bovine bone, and biphasic ceramic made of hydroxyapatite and β-tricalcium phosphate. In vivo study in rats. Lasers Surg. Med. 2018, 50, 651–660. [Google Scholar] [CrossRef]
- Peplow, P.; Chung, T.; Baxter, G. Laser photobiomodulation of wound healing: A review of experimental studies in mouse and rat animal models. Photomed. Laser Surg. 2010, 28, 291–325. [Google Scholar] [CrossRef]
- Bayat, M.; Virdi, A.; Jalalifirouzkouhi, R.; Rezaei, F. Comparison of effects of LLLT and LIPUS on fracture healing in animal models and patients: A systematic review. Prog. Biophys. Mol. Biol. 2018, 132, 3–22. [Google Scholar] [CrossRef]
- Sigurdsson, T.; Lee, M.; Kubota, K.; Turek, T.; Wozney, J.; Wikesjö, U. Periodontal repair in dogs: Recombinant bone morphogenetic protein—2 Significantly enhances periodontal regeneration. J. Periodontol. 1995, 66, 131–138. [Google Scholar] [CrossRef]
- Ribeiro, D.A.; Matsumoto, M.A. Low-level laser therapy improves bone repair in rats treated with anti-inflammatory drugs. J. Oral Rehabil. 2008, 35, 925–933. [Google Scholar] [CrossRef]
- Nascimento, S.B.; Cardoso, C.A.; Ribeiro, T.P.; Almeida, J.D.; Albertini, R.; Munin, E.; Arisawa, E.A.L. Effect of low-level laser therapy and calcitonin on bone repair in castrated rats: A densitometric study. Photomed. Laser Surg. 2010, 28, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Barbosa Pinheiro, A.; Limeira Júnior, F.; Márquez Gerbi, M.; Pedreira Ramalho, L.; Marzola, C.; Carneiro Ponzi, E.; Oliveira Soares, A.; Bandeira De Carvalho, L.; Vieira Lima, H.; Oliveira Gonçalves, T. Effect of 830-nm laser light on the repair of bone defects grafted with inorganic bovine bone and decalcified cortical osseous membrane. J. Clin. Laser Med. Surg. 2003, 21, 383–388. [Google Scholar] [CrossRef]
- Queiroga, A. Evaluation of Bone Repair in the Femur of Rats Submitted to Laser Therapy in Different Wavelengths: An Image Segmentation Method of Analysis. Laser Phys. 2008, 18, 1087–1091. [Google Scholar] [CrossRef]
- De Souza Merli, L.A.; De Medeiros, V.P.; Toma, L.; Reginato, R.D.; Katchburian, E.; Nader, H.B.; Faloppa, F. The low level laser therapy effect on the remodeling of bone extracellular matrix. Photochem. Photobiol. 2012, 88, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Santinoni, C. dos S.; Oliveira, H.F.F.; Batista, V.E. de S.; Lemos, C.A.A.; Verri, F.R. Influence of low-level laser therapy on the healing of human bone maxillofacial defects: A systematic review. J. Photochem. Photobiol. B Biol. 2017, 169, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bölükbas, S.; Greve, T.; Biancosino, C.; Eberlein, M.; Schumacher, S.; Gödde, D.; Störkel, S.; Redwan, B. Diode-pumped neodymium:yttrium aluminum garnet laser effects on the visceral pleura in an ex vivo porcine lung model. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Medalha, C.C.; Santos, A.L.; Veronez Sde, O.; Fernandes, K.R.; Magri, A.M.; Renno, A.C. Low level laser therapy accelerates bone healing in spinal cord injured rats. J. Photochem. Photobiol. 2016, 159, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Babuccu, C.; Keklikoglu, N.; Baydogan, M.; Kaynar, A. Cumulative effect of low-level laser therapy and low-intensity pulsed ultrasound on bone repair in rats. Int. Assoc. Oral Maxillofac. Surg. 2014, 43, 769–776. [Google Scholar] [CrossRef]
- Raina, B.; Qayoom, I.; Larsson, D.; Zheng, M.; Kumar, A.; Isaksson, H.; Lidgren, L.; Tägil, M. Guided tissue engineering for healing of cancellous and cortical bone using a combination of biomaterial based scaffolding and local bone active molecule delivery. Biomaterials 2019, 188, 38–49. [Google Scholar] [CrossRef]





| BM + PBMT | BM + HFB | BM + HFB + PBMT | |
|---|---|---|---|
| 14 days | 22.20 ± 1.77 Aab | 20.00 ± 1.87 Ab | 26.40 ± 1.03 Aa |
| 42 days | 33.20 ± 2.18 Bab | 31.60 ± 1.33 Bb | 38.20 ± 1.59 Ba |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosso, M.P.d.O.; Oyadomari, A.T.; Pomini, K.T.; Della Coletta, B.B.; Shindo, J.V.T.C.; Ferreira Júnior, R.S.; Barraviera, B.; Cassaro, C.V.; Buchaim, D.V.; Teixeira, D.d.B.; et al. Photobiomodulation Therapy Associated with Heterologous Fibrin Biopolymer and Bovine Bone Matrix Helps to Reconstruct Long Bones. Biomolecules 2020, 10, 383. https://doi.org/10.3390/biom10030383
Rosso MPdO, Oyadomari AT, Pomini KT, Della Coletta BB, Shindo JVTC, Ferreira Júnior RS, Barraviera B, Cassaro CV, Buchaim DV, Teixeira DdB, et al. Photobiomodulation Therapy Associated with Heterologous Fibrin Biopolymer and Bovine Bone Matrix Helps to Reconstruct Long Bones. Biomolecules. 2020; 10(3):383. https://doi.org/10.3390/biom10030383
Chicago/Turabian StyleRosso, Marcelie Priscila de Oliveira, Aline Tiemi Oyadomari, Karina Torres Pomini, Bruna Botteon Della Coletta, João Vitor Tadashi Cosin Shindo, Rui Seabra Ferreira Júnior, Benedito Barraviera, Claudia Vilalva Cassaro, Daniela Vieira Buchaim, Daniel de Bortoli Teixeira, and et al. 2020. "Photobiomodulation Therapy Associated with Heterologous Fibrin Biopolymer and Bovine Bone Matrix Helps to Reconstruct Long Bones" Biomolecules 10, no. 3: 383. https://doi.org/10.3390/biom10030383