Maytenus disticha Extract and an Isolated β-Dihydroagarofuran Induce Mitochondrial Depolarization and Apoptosis in Human Cancer Cells by Increasing Mitochondrial Reactive Oxygen Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction of Maytenus disticha
2.3. Cell Culture
2.4. Cytotoxic Assay and IC50 Determination
2.5. Mitochondrial Superoxide and Celllular ROS Determination
2.6. Mitochondrial Membrane Potential Analysis
2.7. Real-Time PCR for Pro-Apoptotic and Anti-Apoptotic Gene Expression Analysis
2.8. Detection of Apoptosis by Flow Cytometry Using Annexin V/PI Assay
2.9. DAPI Staining to Visualize Apoptosis
2.10. Statistical Analyses
3. Results
3.1. Chemical Characterization of Compounds Purified from MD Extract
3.2. X-Ray Structure Analysis of MD-6
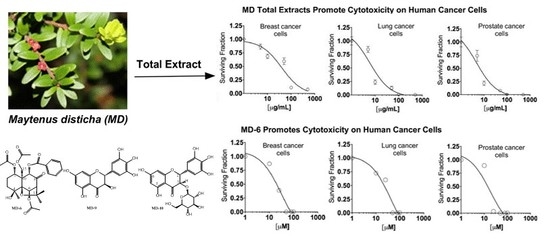
3.3. MD Total Extracts Promote Cytotoxicity on Human Cancer Cells
3.4. MD Total Extracts Increase the BAX/BCL-2 Ratio in Prostate, Breast, and Lung Cancer Cells
3.5. MD Total Extract Promotes a Mitochondrial Membrane Potential Depolarization
3.6. The β-Dihydroagarofuran-Type Sesquiterpene, MD-6, Promotes Cytotoxicity on Human Cancer Cells
3.7. MD and MD-6 Induce Mitochondrial Oxidative Stress in Prostate, Breast, and Lung Cancer Cells
3.8. MD and MD-6 Induce Apoptosis in Prostate, Breast, and Lung Cancer Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Goto, E.; Hosomi, M.; Nishihara, M.; Goto, M.; Yoshida, M.; Kii, T.; Kuwakado, S.; Nishitani, H.; Kawaharada, T.; Takiuchi, H. [Comparison of chemotherapy side effects between elderly and young subjects]. Gan To Kagaku Ryoho. 2012, 39, 2527–2531. [Google Scholar]
- Khazir, J.; Riley, D.L.; Pilcher, L.A.; De-Maayer, P.; Mir, B.A. Anticancer agents from diverse natural sources. Nat. Prod. Commun. 2014, 9, 1655–1669. [Google Scholar] [CrossRef]
- Hartwell, J.L. Plants used against cancer. A survey. Lloydia 1967, 34, 386–425. [Google Scholar]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Li, S.; Zu, Y.; Yang, G.; Yang, Z.; Luo, M.; Jiang, S.; Wink, M.; Efferth, T. Medicinal chemistry of paclitaxel and its analogues. Curr. Med. Chem. 2009, 16, 3966–3985. [Google Scholar] [CrossRef] [PubMed]
- Duflos, A.; Kruczynski, A.; Barret, J.M. Novel aspects of natural and modified vinca alkaloids. Curr. Med. Chem. Anticancer. Agents 2002, 2, 55–70. [Google Scholar] [CrossRef]
- Moudi, M.; Go, R.; Yien, C.Y.; Nazre, M. Vinca alkaloids. Int. J. Prev. Med. 2013, 4, 1231–1235. [Google Scholar] [PubMed]
- Spivey, A.C.; Weston, M.; Woodhead, S. Celastraceae sesquiterpenoids: Biological activity and synthesis. Chem. Soc. Rev. 2002, 31, 43–59. [Google Scholar] [CrossRef]
- Alarcon, J.; Cespedes, C.L.; Munoz, E.; Balbontin, C.; Valdes, F.; Gutierrez, M.; Astudillo, L.; Seigler, D.S. Dihydroagarofuranoid Sesquiterpenes as Acetylcholinesterase Inhibitors from Celastraceae Plants: Maytenus disticha and Euonymus japonicus. J. Agric. Food Chem. 2015, 63, 10250–10256. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, S.; Yin, M.; Wang, X.; Wang, Q.; Shan, Y.; Chen, Y.; Liu, F.; Guo, S.; Feng, X. Six new dihydro-β-agarofuran sesquiterpenes from the stems and leaves of Monimopetalum chinense and their antimicrobial activities. Phytochem. Lett. 2018, 27, 160–166. [Google Scholar] [CrossRef]
- Zhou, J.; Han, N.; Lv, G.; Jia, L.; Liu, Z.; Yin, J. Two New beta-Dihydroagarofuran Sesquiterpenes from Celastrus orbiculatus Thunb and Their Anti-Proliferative Activity. Molecules 2017, 22, 948. [Google Scholar] [CrossRef] [Green Version]
- Callies, O.; Sanchez-Canete, M.P.; Gamarro, F.; Jimenez, I.A.; Castanys, S.; Bazzocchi, I.L. Restoration of Chemosensitivity in P-Glycoprotein-Dependent Multidrug-Resistant Cells by Dihydro-beta-agarofuran Sesquiterpenes from Celastrus vulcanicola. J. Nat. Prod. 2015, 78, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Momtaz, S.; Hussein, A.A.; Ostad, S.N.; Abdollahi, M.; Lall, N. Growth inhibition and induction of apoptosis in human cancerous HeLa cells by Maytenus procumbens. Food Chem. Toxicol. 2013, 51, 38–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.; Qi, Z.; Li, Q.; Wu, W. Validation of the Target Protein of Insecticidal Dihydroagarofuran Sesquiterpene Polyesters. Toxins 2016, 8, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Li, D.; Xi, X.; Liu, L.; Zhao, X.; Wu, W.; Zhang, J. Molecular Insights into the Potential Insecticidal Interaction of beta-Dihydroagarofuran Derivatives with the H Subunit of V-ATPase. Molecules 2017, 22, 1701. [Google Scholar] [CrossRef]
- Boland, M.L.; Chourasia, A.H.; Macleod, K.F. Mitochondrial dysfunction in cancer. Front. Oncol. 2013, 3, 292. [Google Scholar] [CrossRef] [Green Version]
- Min, H.Y.; Jang, H.J.; Park, K.H.; Hyun, S.Y.; Park, S.J.; Kim, J.H.; Son, J.; Kang, S.S.; Lee, H.Y. The natural compound gracillin exerts potent antitumor activity by targeting mitochondrial complex II. Cell Death Dis. 2019, 10, 810. [Google Scholar] [CrossRef] [Green Version]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive Oxygen Species in the Tumor Microenvironment: An Overview. Cancers 2019, 11, 1191. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXS-97, Program for Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Flack, H.D. On enantiomorph-polarity estimation. Acta Crystallogr. Sect. A 1983, 39, 876–881. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP III for Windows. J. Appl. Cryst 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Brandenburg, K.; Putz, H. Diamond: Crystal and Molecular Structure Visualization; Crystal Impact: Bonn, Germany, 2008. [Google Scholar]
- Paz, C.; von Dossow, D.; Tiznado, V.; Suarez, S.; Cukiernik, F.D.; Baggio, R. A dihydro-beta-agarofuran sesquiterpene from Maytenus boaria. Acta Crystallogr. C Struct. Chem. 2017, 73, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Paz, C.; Heydenreich, M.; Schmidt, B.; Vadra, N.; Baggio, R. Three new dihydro-beta-agarofuran sesquiterpenes from the seeds of Maytenus boaria. Acta Crystallogr. C Struct. Chem. 2018, 74, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Perestelo, N.R.; Jimenez, I.A.; Tokuda, H.; Vazquez, J.T.; Ichiishi, E.; Bazzocchi, I.L. Absolute Configuration of Dihydro-beta-agarofuran Sesquiterpenes from Maytenus jelskii and Their Potential Antitumor-Promoting Effects. J. Nat. Prod. 2016, 79, 2324–2331. [Google Scholar] [CrossRef]
- Wibowo, M.; Levrier, C.; Sadowski, M.C.; Nelson, C.C.; Wang, Q.; Holst, J.; Healy, P.C.; Hofmann, A.; Davis, R.A. Bioactive Dihydro-beta-agarofuran Sesquiterpenoids from the Australian Rainforest Plant Maytenus bilocularis. J. Nat. Prod. 2016, 79, 1445–1453. [Google Scholar] [CrossRef] [Green Version]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arraez-Roman, D.; Segura-Carretero, A. HPLC-DAD-ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Dong, M.; He, X.; Liu, R.H. Phytochemicals of black bean seed coats: Isolation, structure elucidation, and their antiproliferative and antioxidative activities. J. Agric. Food Chem. 2007, 55, 6044–6051. [Google Scholar] [CrossRef]
- Garcia, M.E.; Motrich, R.D.; Caputto, B.L.; Sanchez, M.; Palermo, J.A.; Estevez-Braun, A.; Ravelo, A.G.; Nicotra, V.E. Agarofuran sesquiterpenes from Schaefferia argentinensis. Phytochemistry 2013, 94, 260–267. [Google Scholar] [CrossRef]
- Luo, G.Q.; Zeng, S.; Liu, D.Y. [Inhibitory effects of ampelopsin on angiogenesis]. Zhong Yao Cai 2006, 29, 146–150. [Google Scholar]
- Qi, S.; Xin, Y.; Guo, Y.; Diao, Y.; Kou, X.; Luo, L.; Yin, Z. Ampelopsin reduces endotoxic inflammation via repressing ROS-mediated activation of PI3K/Akt/NF-kappaB signaling pathways. Int. Immunopharmacol. 2012, 12, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.L.; Tong, Q.; Wang, W.Q.; Shi, C.Y.; Xiong, W.; Chen, J.; Liu, X.; Fang, J.G. Suppression of Inflammatory Responses by Dihydromyricetin, a Flavonoid from Ampelopsis grossedentata, via Inhibiting the Activation of NF-kappaB and MAPK Signaling Pathways. J. Nat. Prod. 2015, 78, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Lindemeyer, A.K.; Gonzalez, C.; Shao, X.M.; Spigelman, I.; Olsen, R.W.; Liang, J. Dihydromyricetin as a novel anti-alcohol intoxication medication. J. Neurosci. 2012, 32, 390–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabbir, M.; Syed, D.N.; Lall, R.K.; Khan, M.R.; Mukhtar, H. Potent anti-proliferative, pro-apoptotic activity of the Maytenus royleanus extract against prostate cancer cells: Evidence in in-vitro and in-vivo models. PLoS ONE 2015, 10, e0119859. [Google Scholar] [CrossRef]
- Araujo Junior, R.F.; Oliveira, A.L.; Pessoa, J.B.; Garcia, V.B.; Guerra, G.C.; Soares, L.A.; Souza, T.P.; Petrovick, P.R.; Araujo, A.A. Maytenus ilicifolia dry extract protects normal cells, induces apoptosis and regulates Bcl-2 in human cancer cells. Exp. Biol. Med. 2013, 238, 1251–1258. [Google Scholar] [CrossRef]
- Gonzalez, A.G.; Jimenez, I.A.; Nunez, M.P.; Ravelo, A.G.; Bazzocchi, I.L.; Munoz, O.M.; Aguilar, M.A. New sesquiterpenes fromMaytenus species (Celastraceae). Taxonomic and chemotaxonomic considerations concerning chilean Maytenus. J. Chem. Ecol. 1994, 20, 823–830. [Google Scholar] [CrossRef]
- Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria as targets for cancer chemotherapy. Semin. Cancer Biol. 2009, 19, 57–66. [Google Scholar] [CrossRef]
- Ott, M.; Robertson, J.D.; Gogvadze, V.; Zhivotovsky, B.; Orrenius, S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc. Natl. Acad. Sci. USA 2002, 99, 1259–1263. [Google Scholar] [CrossRef] [Green Version]











© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Chavarría, I.; Duprat, F.; Roa, F.J.; Jara, N.; Toledo, J.R.; Miranda, F.; Becerra, J.; Inostroza, A.; Kelling, A.; Schilde, U.; et al. Maytenus disticha Extract and an Isolated β-Dihydroagarofuran Induce Mitochondrial Depolarization and Apoptosis in Human Cancer Cells by Increasing Mitochondrial Reactive Oxygen Species. Biomolecules 2020, 10, 377. https://doi.org/10.3390/biom10030377
González-Chavarría I, Duprat F, Roa FJ, Jara N, Toledo JR, Miranda F, Becerra J, Inostroza A, Kelling A, Schilde U, et al. Maytenus disticha Extract and an Isolated β-Dihydroagarofuran Induce Mitochondrial Depolarization and Apoptosis in Human Cancer Cells by Increasing Mitochondrial Reactive Oxygen Species. Biomolecules. 2020; 10(3):377. https://doi.org/10.3390/biom10030377
Chicago/Turabian StyleGonzález-Chavarría, Iván, Felix Duprat, Francisco J. Roa, Nery Jara, Jorge R. Toledo, Felipe Miranda, José Becerra, Alejandro Inostroza, Alexandra Kelling, Uwe Schilde, and et al. 2020. "Maytenus disticha Extract and an Isolated β-Dihydroagarofuran Induce Mitochondrial Depolarization and Apoptosis in Human Cancer Cells by Increasing Mitochondrial Reactive Oxygen Species" Biomolecules 10, no. 3: 377. https://doi.org/10.3390/biom10030377