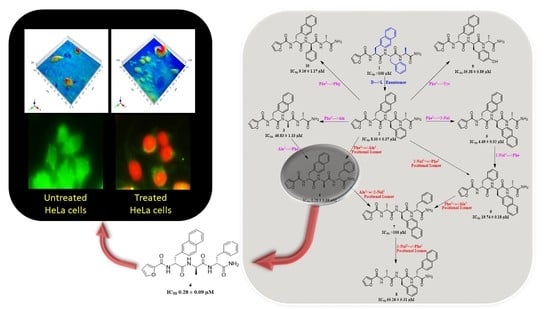
Furan-Conjugated Tripeptides as Potent Antitumor Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Experimental Procedure for Synthesis of Furan-Conjugated Tripeptides
- Conjugate 1: Purple amorphous solid, 82.06 mg (51.7%); mp, 214 °C; UV (ACN) λmax (log ε) 252 (3.85) nm; IR (KBr, cm−1) 3299 (NH), 3071 (=C-H), 2974 (C-H), 1645 (-NHC=O stretching), 1543 (Aromatic -C=C-, CN stretching, NH bending), 1424 (C-H bending, Aromatic -C=C- stretching), and 1208 (C-O stretching, CN stretching, NH bending); 1H-NMR (DMSO-d6, 600 MHz) and 13C-NMR (DMSO-d6, 150 MHz) see Table S1.
- Conjugate 2: White amorphous solid, 50.04 mg (31.5%); mp 212–213 °C; UV (ACN) λmax (log ε) 252 (4.15) nm; IR (KBr, cm−1) 3265 (NH), 3058 (=C-H), 2931 (C-H), 1641 (-NHC=O stretching), 1533 (Aromatic -C=C-, CN stretching, NH bending), 1471 (C-H bending, Aromatic -C=C- stretching), and 1201 (C-O stretching, CN stretching, NH bending); 1H-NMR (DMSO-d6, 600 MHz) and 13C-NMR (DMSO-d6, 150 MHz) see Table S1.
- Conjugate 3: White amorphous solid, 81.5 mg (60.0%); mp 212–213 °C; UV (ACN) λmax (log ε) 250 (4.14) nm; IR (KBr, cm−1) 3300 (NH), 30,791 (=C-H), 3011 (C-H), 1633 (-NHC=O stretching), 1546 (Aromatic -C=C-, CN stretching, NH bending), 1423 (C-H bending, Aromatic -C=C- stretching), and 1218 (C-O stretching, CN stretching, NH bending); 1H-NMR (DMSO-d6, 600 MHz) and 13C-NMR (DMSO-d6, 150 MHz) see Table S1.
- Conjugate 4: Pale yellow amorphous solid, 80.17 mg (50.5%); mp, 173 °C; UV (ACN) λmax (log ε) 225 (4.12) and 254 (3.42) nm; IR (KBr, cm−1) 3299 (NH), 3086 (=C-H), 2972 (C-H), 1643 (-NHC=O stretching), 1544 (Aromatic -C=C-, CN stretching, NH bending), and 1215 (C-O stretching, CN stretching, NH bending); 1H-NMR (DMSO-d6, 600 MHz) and 13C-NMR (DMSO-d6, 150 MHz) see Table S1.
- Conjugate 5: Purple amorphous solid, 82.99 mg (47.7%); mp, 205 °C; UV (ACN) λmax (log ε) 256 (3.62) nm; IR (KBr, cm−1) 3303 (NH), 3075 (=C-H), 2989 (C-H), 1680, 1649 (-NHC=O stretching), 1544 (Aromatic -C=C-, CN stretching, NH bending), 1433 (C-H bending, Aromatic -C=C- stretching), and 1213 (C-O stretching, CN stretching, NH bending); 1H-NMR (DMSO-d6, 600 MHz) and 13C-NMR (DMSO-d6, 150 MHz) see Table S1.
- Conjugate 6: Purple amorphous solid, 69.4 mg (43.7%); mp 199-206 °C; UV (ACN) λmax (log ε) 252 (4.18) nm; IR (KBr, cm−1) 3298 (NH), 3068 (=C-H), 2958 (C-H), 1680, 1645 (-NHC=O stretching), 1543 (Aromatic -C=C-, CN stretching, NH bending), 1436 (C-H bending, Aromatic -C=C- stretching), and 1214 (C-O stretching, CN stretching, NH bending); 1H-NMR (DMSO-d6, 600 MHz) and 13C-NMR (DMSO-d6, 150 MHz) see Table S2.
- Conjugate 7: White amorphous solid, 60.3 mg (37.9%); mp, 135 °C; UV (ACN) λmax (log ε) 256 (4.04) nm; IR (KBr, cm−1) 3294 (NH), 3057 (=C-H), 2917 (C-H), 1670, 1633 (-NHC=O stretching), and 1528 (Aromatic -C=C-, CN stretching, NH bending); 1H-NMR (DMSO-d6, 600 MHz) and 13C-NMR (DMSO-d6, 150 MHz) see Table S2.
- Conjugate 8: White amorphous solid, 51.56 mg (32.4%); mp, 237 °C; UV (ACN) λmax (log ε) 252 (4.09) nm; IR (KBr, cm−1) 3296 (NH), 3075 (=C-H), 2950 (C-H), 1677, 1643 (-NHC=O stretching), 1546 (Aromatic -C=C-, CN stretching, NH bending), 1426 (C-H bending, Aromatic -C=C- stretching), and 1227 (C-O stretching, CN stretching, NH bending); 1H-NMR (DMSO-d6, 600 MHz) and 13C-NMR (DMSO-d6, 150 MHz) see Table S2.
- Conjugate 9: Grey amorphous solid, 85.95 mg (52.5%); mp 194–199 °C; UV (ACN) λmax (log ε) 254 (4.24) nm; IR (KBr, cm−1) 3398 (NH), 3304 (OH), 3094 (=C-H), 2989 (C-H), 1676, 1650 (-NHC=O stretching), 1538 (Aromatic -C=C-, CN stretching, NH bending), 1431 (C-H bending, Aromatic -C=C- stretching), and 1212 (C-O stretching, CN stretching, NH bending); 1H-NMR (DMSO-d6, 600 MHz) and 13C-NMR (DMSO-d6, 150 MHz) see Table S2.
- Conjugate 10: Brown amorphous solid, 72.43 mg (46.8%); mp, 220 °C; UV (ACN) λmax (log ε) 249 (4.07) nm; IR (KBr, cm−1) 3301 (NH), 3055 (=C-H), 2935 (C-H), 1634 (-NHC=O stretching), 1512 (Aromatic -C=C-, CN stretching, NH bending), 1452 (C-H bending, Aromatic -C=C- stretching), and 1204 (C-O stretching, CN stretching, NH bending); 1H-NMR (DMSO-d6, 600 MHz) and 13C-NMR (DMSO-d6, 150 MHz) see Table S2.
2.3. Anti-Proliferative Assay
2.4. Atomic Force Microscopy of HeLa Cells
2.5. Rhodamine 123 and Propidium Iodide Staining Assay
2.6. Synthesis of Dendrimeric Conjugate 4a
2.7. Synthesis of Dendrimeric Conjugate 4b
3. Results
Mechanistic Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Patents
References
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [Green Version]
- Vu, M.; Yu, J.; Awolude, O.A.; Chuang, L. Cervical cancer worldwide. Curr. Probl. Cancer 2018, 42, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.A.; Sajjad, S.; Malik, H. Cervical cancer in Pakistan: A review. J. Pak. Med. Assoc. 2017, 67, 1074–1077. [Google Scholar] [PubMed]
- Li, L.; Mok, H.; Jhaveri, P.; Bonnen, M.D.; Sikora, A.G.; Eissa, N.T.; Komaki, R.U.; Ghebre, Y.T. Anticancer therapy and lung injury: Molecular mechanisms. Expert Rev. Anticancer Ther. 2018, 18, 1041–1057. [Google Scholar] [CrossRef] [PubMed]
- Reinert, T.; Baldotto, C.S.R.; Nunes, F.A.P.; Scheliga, A.A.S. Bleomycin-induced lung injury. J. Cancer Res. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef]
- Cohen, S.J.; Alpaugh, R.K.; Gross, S.; O’Hara, S.M.; Smirnov, D.A.; Terstappen, L.W.; Allard, W.J.; Bilbee, M.; Cheng, J.D.; Hoffman, J.P.; et al. Isolation and characterization of circulating tumor cells in patients with metastatic colorectal cancer. Clin. Colorectal Cancer 2006, 6, 125–132. [Google Scholar] [CrossRef]
- Thundimadathil, J. Cancer treatment using peptides: Current therapies and future prospects. J. Amino Acids 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Finan, B.; Mayer, J.P.; DiMarchi, R.D. Peptide conjugates with small molecules designed to enhance efficacy and safety. Molecules 2019, 24, 1855. [Google Scholar] [CrossRef] [Green Version]
- Aungst, B.J. Intestinal permeation enhancers. J. Pharm. Sci. 2000, 89, 429–442. [Google Scholar] [CrossRef]
- Banga, A.K.; Chien, Y.W. Systemic delivery of therapeutic peptides and proteins. Int. J. Pharm. 1988, 48, 15–50. [Google Scholar] [CrossRef]
- Pauletti, G.M.; Gangwar, S.; Knipp, G.T.; Nerurkar, M.M.; Okumu, F.W.; Tamura, K.; Siahaan, T.J.; Borchardt, R.T. Structural requirements for intestinal absorption of peptide drugs. J. Control. Release 1996, 41, 3–17. [Google Scholar] [CrossRef]
- Mazandaran, K.E.; Mirshokraee, S.A.; Didehban, K.; Tehrani, M.H.H. Design, synthesis and biological evaluation of Ciprofloxacin-peptide conjugates as anticancer agents. Iran. J. Pharm. Res. IJPR 2019, 18, 1823. [Google Scholar]
- Li, Y.; Zheng, X.; Gong, M.; Zhang, J. Delivery of a peptide-drug conjugate targeting the blood brain barrier improved the efficacy of paclitaxel against glioma. Oncotarget 2016, 7, 79401. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Jiang, M.; Wang, H.; Fu, H. Installing amino acids and peptides on N-heterocycles under visible-light assistance. Sci. Rep. 2016, 6, 20068. [Google Scholar] [CrossRef] [Green Version]
- Suresha, G.; Prakasha, K.; Kumara, K.S.; Kapfo, W.; Gowda, D.C. Design and synthesis of heterocyclic conjugated peptides as novel antimicrobial agents. Int. J. Pept. Res. Ther. 2009, 15, 25–30. [Google Scholar] [CrossRef]
- Moore, P.A.; Hersh, E.V. Celecoxib and rofecoxib: The role of COX-2 inhibitors in dental practice. J. Am. Dent. Assoc. 2001, 132, 451–456. [Google Scholar] [CrossRef]
- Ryan, W.G.; Moldave, K.; Carithers, D. Clinical effectiveness and safety of a new NSAID, firocoxib: A 1000 dog study. Vet. Ther. 2006, 7, 119. [Google Scholar]
- Gao, F.; Xiao, J.; Huang, G. Current scenario of tetrazole hybrids for antibacterial activity. Eur. J. Med. Chem. 2019, 184, 111744. [Google Scholar] [CrossRef]
- Wang, M.; Rakesh, K.P.; Leng, J.; Fang, W.-Y.; Ravindar, L.; Channe, G.D.; Qin, H.-L. Amino acids/peptides conjugated heterocycles: A tool for the recentdevelopment of novel therapeutic agents. Bioorg. Chem. 2018, 7, 113–129. [Google Scholar] [CrossRef]
- Oliva, P.; Romagnoli, R.; Manfredini, S.; Brancale, A.; Ferla, S.; Hamel, E.; Ronca, R.; Maccarinelli, F.; Giacomini, A.; Rruga, F. Design, synthesis, in vitro and in vivo biological evaluation of 2-amino-3-aroylbenzo [b] furan derivatives as highly potent tubulin polymerization inhibitors. Eur. J. Med. Chem. 2020, 112448. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Xu, B.; Niu, Y.; Xu, F.; Liang, L.; Wang, C.; Yu, J.; Yan, G.; Wang, W.; Jin, H. Synthesis, bioactivity, docking and molecular dynamics studies of furan-based peptides as 20S proteasome inhibitors. ChemMedChem 2015, 10, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Behnam, M.A.; Graf, D.; Bartenschlager, R.; Zlotos, D.P.; Klein, C.D. Discovery of nanomolar dengue and West Nile virus protease inhibitors containing a 4-benzyloxyphenylglycine residue. J. Med. Chem. 2015, 58, 9354–9370. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, G.; Gentilucci, L.; Tolomelli, A. Unusual Amino Acids—Synthesis and Introduction into Naturally Occurring Peptides and Biologically Active Analogues. Mini Rev. Med. Chem. 2006, 6, 293–304. [Google Scholar] [CrossRef]
- Evans, B.J.; King, A.T.; Katsifis, A.; Matesic, L.; Jamie, J.F. Methods to Enhance the Metabolic Stability of Peptide-Based PET Radiopharmaceuticals. Molecules 2020, 25, 231. [Google Scholar] [CrossRef]
- Groß, A.; Hashimoto, C.; Sticht, H.; Eichler, J. Synthetic Peptides as Protein Mimics. Front Bioeng. Biotechnol. 2016, 3, 211. [Google Scholar] [CrossRef] [Green Version]
- Olleik, H.; Baydoun, E.; Perrier, J.; Hijazi, A.; Raymond, J.; Manzoni, M.; Dupuis, L.; Pauleau, G.; Goudard, Y.; de La Villéon, B. Temporin-SHa and Its Analogs as Potential Candidates for the Treatment of Helicobacter pylori. Biomolecules 2019, 9, 598. [Google Scholar] [CrossRef] [Green Version]
- Ngenge, T.A.; Jabeen, A.; Maurice, T.F.; Baig, T.A.; Shaheen, F. Organic and Mineral Composition of Seeds of Afrostyrax lepidophyllus Mildbr. and Evaluation of ROS Inhibition and Cytotoxicity of Isolated Compounds. Chem. Afr. 2019, 2, 615–624. [Google Scholar] [CrossRef] [Green Version]
- Ogunlakin, A.D.; Sonibare, M.A.; Jabeen, A.; Shaheen, F.; Shah, S.F. Antiproliferative and ameliorative effects of Tetracera potatoria and its constituent. Adv. Tradit. Med. 2020. [Google Scholar] [CrossRef]
- Vega, S.C.; Martínez, D.A.; Chalá, M.S.; Vargas, H.A.; Rosas, J.E. Design, synthesis and evaluation of branched RRWQWR-based peptides as antibacterial agents against clinically relevant gram-positive and gram-negative pathogens. Front. Microbiol. 2018, 9, 329. [Google Scholar] [CrossRef] [Green Version]
- Townsend, J.B.; Shaheen, F.; Liu, R.; Lam, K.S. Jeffamine derivatized TentaGel beads and poly (dimethylsiloxane) microbead cassettes for ultrahigh-throughput in situ releasable solution-phase cell-based screening of one-bead-one-compound combinatorial small molecule libraries. J. Comb. Chem. 2010, 12, 700–712. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.S.; Cho, C.H.; Park, E.K.; Jung, M.-H.; Yoon, K.-S.; Park, H.-K. AFM-detected apoptotic changes in morphology and biophysical property caused by paclitaxel in Ishikawa and HeLa cells. PLoS ONE 2012, 7, e30066. [Google Scholar] [CrossRef] [Green Version]






| Conjugate | Sequence | [M+H]+Calculated | [M+H]+Found | Chemical Formula | tR * (min) | IC50 ± SD (µg/mL) | IC50 ± SD (µM) |
|---|---|---|---|---|---|---|---|
| 1 | 2-Fur4-D-2-Nal3-D-Phe2-D-Ala1-CONH2 | 527.2294 | 527.2305 | C30H31N4O5 | 3.68 | >100 | >100 |
| 2 | 2-Fur4-L-2-Nal3-L-Phe2-L-Ala1-CONH2 | 527.2294 | 527.2305 | C30H31N4O5 | 3.741 | 4.40 ± 0.20 | 8.35 ± 0.37 |
| 3 | 2-Fur4-L-2-Nal3-L-Ala2-L-Ala1-CONH2 | 451.1981 | 451.198 | C24H27N4O5 | 3.289 | 21.1 ± 0.60 | 46.83 ± 1.33 |
| 4 | 2-Fur4-L-2-Nal3-L-Ala2-L-Phe1-CONH2 | 527.2294 | 527.2299 | C30H31N4O5 | 3.711 | 0.15 ± 0.05 | 0.28 ± 0.09 |
| 5 | 2-Fur4-L-2-Nal3-L-2-Nal2-L-Ala1-CONH2 | 577.2451 | 577.2472 | C34H33N4O5 | 3.857 | 2.59 ± 0.30 | 4.49 ± 0.52 |
| 6 | 2-Fur4-L-Phe3-L-2-Nal2-L-Ala1-CONH2 | 527.2294 | 527.2309 | C30H31N4O5 | 3.685 | 10.4±0.10 | 19.74 ± 0.18 |
| 7 | 2-Fur4-L-Ala3-L-2-Nal2-L-Phe1-CONH2 | 527.2294 | 527.2311 | C30H31N4O5 | 3.706 | >100 | >100 |
| 8 | 2-Fur4-L-Ala3-L-Phe2-L-2-Nal1-CONH2 | 527.2294 | 527.2269 | C30H31N4O5 | 3.759 | 29.1 ± 2.8 | 55.26 ± 5.31 |
| 9 | 2-Fur4-L-2-Nal3-L-Tyr2-L-Ala1-CONH2 | 543.2244 | 543.2256 | C30H31N4O6 | 3.266 | 19.2 ± 3.2 | 35.38 ± 5.89 |
| 10 | 2-Fur4-L-2-Nal3-L-Phg2-L-Ala1-CONH2 | 513.2138 | 513.2131 | C29H29N4O5 | 3.483 | 4.70 ± 0.60 | 9.16 ± 1.17 |
| IC50 (µg/mL) | ||||||
|---|---|---|---|---|---|---|
| HeLa | HUVEC | IMR-90 | MCF-7 | MDA-MB-231 | 3T3 | |
| Conjugate 4 | 0.15 ± 0.05 | >100 | >100 | >100 | >100 | >100 |
| Doxorubicin | 3.09 ± 0.16 | 0.02 ± 0.005 | 0.06 ± 0.01 | 0.01 ± 0.005 | 0.21 ± 0.03 | <1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, H.; Jabeen, A.; Maharjan, R.; Nadeem-ul-Haque, M.; Aamra, H.; Nazir, S.; Khan, S.; Olleik, H.; Maresca, M.; Shaheen, F. Furan-Conjugated Tripeptides as Potent Antitumor Drugs. Biomolecules 2020, 10, 1684. https://doi.org/10.3390/biom10121684
Ali H, Jabeen A, Maharjan R, Nadeem-ul-Haque M, Aamra H, Nazir S, Khan S, Olleik H, Maresca M, Shaheen F. Furan-Conjugated Tripeptides as Potent Antitumor Drugs. Biomolecules. 2020; 10(12):1684. https://doi.org/10.3390/biom10121684
Chicago/Turabian StyleAli, Hunain, Almas Jabeen, Rukesh Maharjan, Muhammad Nadeem-ul-Haque, Husena Aamra, Salma Nazir, Serab Khan, Hamza Olleik, Marc Maresca, and Farzana Shaheen. 2020. "Furan-Conjugated Tripeptides as Potent Antitumor Drugs" Biomolecules 10, no. 12: 1684. https://doi.org/10.3390/biom10121684