Large Animal Models of Cell-Free Cardiac Regeneration
Abstract
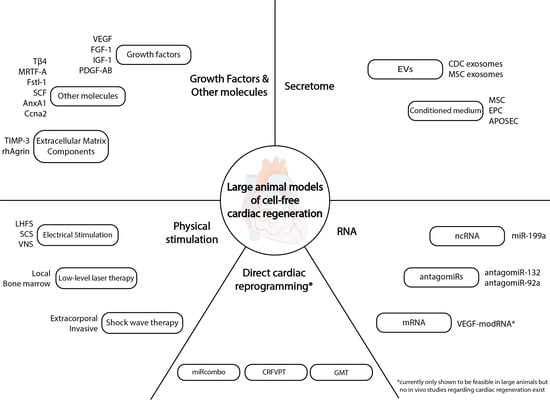
:1. Introduction
2. Cell-Free Cardiac Regeneration Therapies
2.1. Stem Cell Secretomes and Extracellular Vesicles (EVs)
2.1.1. Secretomes
2.1.2. EVs
2.2. RNA-Based Therapies
2.2.1. ncRNAs
2.2.2. Coding RNAs
2.3. Growth Factors and Single Molecules
2.3.1. Growth Factors
2.3.2. Other Molecules
2.4. Physical Stimulation of Regeneration
2.4.1. Shock Wave Therapy (SWT)
2.4.2. Low-Level Laser Therapy (LLLT)
2.4.3. Localized High-Frequency Electrical Stimulation (LHFS)
2.4.4. Bioelectrical Stimulation
2.5. Direct Cardiac Reprogramming
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coats, A.J.S.; Pieske, B.; Linde, C.; Jankowska, E.A.; Ruschitzka, F.; Rutten, F.H.; Rosano, G.M.C.; Bueno, H.; Riley, J.P.; Cleland, J.G.F.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Gyongyosi, M.; Wojakowski, W.; Lemarchand, P.; Lunde, K.; Tendera, M.; Bartunek, J.; Marban, E.; Assmus, B.; Henry, T.D.; Traverse, J.H.; et al. Meta-Analysis of Cell-based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ. Res. 2015, 116, 1346–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.N.; Cores, J.; Huang, K.; Cui, X.L.; Luo, L.; Zhang, J.Y.; Li, T.S.; Qian, L.; Cheng, K. Concise Review: Is Cardiac Cell Therapy Dead? Embarrassing Trial Outcomes and New Directions for the Future. Stem Cells Transl. Med. 2018, 7, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Van Laake, L.W.; Botker, H.E.; Davidson, S.M.; De Caterina, R.; Engel, F.B.; Eschenhagen, T.; Fernandez-Aviles, F.; Hausenloy, D.J.; Hulot, J.S.; et al. ESC Working Group on Cellular Biology of the Heart: Position paper for Cardiovascular Research: Tissue engineering strategies combined with cell therapies for cardiac repair in ischaemic heart disease and heart failure. Cardiovasc. Res. 2019, 115, 488–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madonna, R.; Van Laake, L.W.; Davidson, S.M.; Engel, F.B.; Hausenloy, D.J.; Lecour, S.; Leor, J.; Perrino, C.; Schulz, R.; Ytrehus, K.; et al. Position Paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: Cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur. Heart J. 2016, 37, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.A. A Time to Press Reset and Regenerate Cardiac Stem Cell Biology. JAMA Cardiol. 2019, 4, 95–96. [Google Scholar] [CrossRef]
- Price, E.L.; Vieira, J.M.; Riley, P.R. Model organisms at the heart of regeneration. Dis. Models Mech. 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Bryant, D.M.; O‘Meara, C.C.; Ho, N.N.; Gannon, J.; Cai, L.; Lee, R.T. A systematic analysis of neonatal mouse heart regeneration after apical resection. J. Mol. Cell. Cardiol. 2015, 79, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Darehzereshki, A.; Rubin, N.; Gamba, L.; Kim, J.; Fraser, J.; Huang, Y.; Billings, J.; Mohammadzadeh, R.; Wood, J.; Warburton, D.; et al. Differential regenerative capacity of neonatal mouse hearts after cryoinjury. Dev. Biol. 2015, 399, 91–99. [Google Scholar] [CrossRef]
- Haubner, B.J.; Adamowicz-Brice, M.; Khadayate, S.; Tiefenthaler, V.; Metzler, B.; Aitman, T.; Penninger, J.M. Complete cardiac regeneration in a mouse model of myocardial infarction. Aging 2012, 4, 966–977. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; D’Agostino, G.; Loo, S.J.; Wang, C.X.; Su, L.P.; Tan, S.H.; Tee, G.Z.; Pua, C.J.; Pena, E.M.; Cheng, R.B.; et al. Early Regenerative Capacity in the Porcine Heart. Circulation 2018, 138, 2798–2808. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, E.; Zhao, M.; Chong, Z.; Fan, C.; Tang, Y.; Hunter, J.D.; Borovjagin, A.V.; Walcott, G.P.; Chen, J.Y.; et al. Regenerative Potential of Neonatal Porcine Hearts. Circulation 2018, 138, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Haubner, B.J.; Schneider, J.; Schweigmann, U.; Schuetz, T.; Dichtl, W.; Velik-Salchner, C.; Stein, J.-I.; Penninger, J.M. Functional Recovery of a Human Neonatal Heart After Severe Myocardial Infarction. Circ. Res. 2016, 118, 216–221. [Google Scholar] [CrossRef] [Green Version]
- Saker, D.M.; Walsh-Sukys, M.; Spector, M.; Zahka, K.G. Cardiac recovery and survival after neonatal myocardial infarction. Pediatric Cardiol. 1997, 18, 139–142. [Google Scholar] [CrossRef]
- Vivien, C.J.; Hudson, J.E.; Porrello, E.R. Evolution, comparative biology and ontogeny of vertebrate heart regeneration. NPJ Regen. Med. 2016, 1, 16012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spannbauer, A.; Traxler, D.; Zlabinger, K.; Gugerell, A.; Winkler, J.; Mester-Tonczar, J.; Lukovic, D.; Müller, C.; Riesenhuber, M.; Pavo, N.; et al. Large Animal Models of Heart Failure With Reduced Ejection Fraction (HFrEF). Front. Cardiovasc. Med. 2019, 6, 117. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.G.; Cheng, K.; Marbán, E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2014, 2, 606–619. [Google Scholar] [CrossRef] [Green Version]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Showalter, M.R.; Wancewicz, B.; Fiehn, O.; Archard, J.A.; Clayton, S.; Wagner, J.; Deng, P.; Halmai, J.; Fink, K.D.; Bauer, G.; et al. Primed mesenchymal stem cells package exosomes with metabolites associated with immunomodulation. Biochem. Biophys. Res. Commun. 2019, 512, 729–735. [Google Scholar] [CrossRef]
- Namazi, H.; Mohit, E.; Namazi, I.; Rajabi, S.; Samadian, A.; Hajizadeh-Saffar, E.; Aghdami, N.; Baharvand, H. Exosomes secreted by hypoxic cardiosphere-derived cells enhance tube formation and increase pro-angiogenic miRNA. J. Cell. Biochem. 2018, 119, 4150–4160. [Google Scholar] [CrossRef]
- Beer, L.; Mildner, M.; Gyöngyösi, M.; Ankersmit, H.J. Peripheral blood mononuclear cell secretome for tissue repair. Apoptosis Int. J. Program. Cell Death 2016, 21, 1336–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, E.; Marinaro, F.; de Pedro, M.d.L.Á.; Sánchez-Margallo, F.M.; Gómez-Serrano, M.; Ponath, V.; Pogge von Strandmann, E.; Jorge, I.; Vázquez, J.; Fernández-Pereira, L.M.; et al. The Immunomodulatory Signature of Extracellular Vesicles From Cardiosphere-Derived Cells: A Proteomic and miRNA Profiling. Front. Cell Dev. Biol. 2020, 8, 321. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.R.; Berman, A.E.; Weintraub, N.L.; Tang, Y.L. Electrical stimulation to optimize cardioprotective exosomes from cardiac stem cells. Med. Hypotheses 2016, 88, 6–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmers, L.; Lim, S.K.; Arslan, F.; Armstrong, J.S.; Hoefer, I.E.; Doevendans, P.A.; Piek, J.J.; El Oakley, R.M.; Choo, A.; Lee, C.N.; et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007, 1, 129–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmers, L.; Lim, S.K.; Hoefer, I.E.; Arslan, F.; Lai, R.C.; van Oorschot, A.A.; Goumans, M.J.; Strijder, C.; Sze, S.K.; Choo, A.; et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011, 6, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Hynes, B.; Kumar, A.H.; O’Sullivan, J.; Klein Buneker, C.; Leblond, A.L.; Weiss, S.; Schmeckpeper, J.; Martin, K.; Caplice, N.M. Potent endothelial progenitor cell-conditioned media-related anti-apoptotic, cardiotrophic, and pro-angiogenic effects post-myocardial infarction are mediated by insulin-like growth factor-1. Eur. Heart J. 2013, 34, 782–789. [Google Scholar] [CrossRef]
- Pavo, N.; Zimmermann, M.; Pils, D.; Mildner, M.; Petrási, Z.; Petneházy, Ö.; Fuzik, J.; Jakab, A.; Gabriel, C.; Sipos, W.; et al. Long-acting beneficial effect of percutaneously intramyocardially delivered secretome of apoptotic peripheral blood cells on porcine chronic ischemic left ventricular dysfunction. Biomaterials 2014, 35, 3541–3550. [Google Scholar] [CrossRef] [Green Version]
- Beer, L.; Zimmermann, M.; Mitterbauer, A.; Ellinger, A.; Gruber, F.; Narzt, M.S.; Zellner, M.; Gyöngyösi, M.; Madlener, S.; Simader, E.; et al. Analysis of the Secretome of Apoptotic Peripheral Blood Mononuclear Cells: Impact of Released Proteins and Exosomes for Tissue Regeneration. Sci. Rep. 2015, 5, 16662. [Google Scholar] [CrossRef] [PubMed]
- Lichtenauer, M.; Mildner, M.; Baumgartner, A.; Hasun, M.; Werba, G.; Beer, L.; Altmann, P.; Roth, G.; Gyöngyösi, M.; Podesser, B.K.; et al. Intravenous and intramyocardial injection of apoptotic white blood cell suspensions prevents ventricular remodelling by increasing elastin expression in cardiac scar tissue after myocardial infarction. Basic Res. Cardiol. 2011, 106, 645–655. [Google Scholar] [CrossRef] [Green Version]
- Kishore, R.; Khan, M. More Than Tiny Sacks: Stem Cell Exosomes as Cell-Free Modality for Cardiac Repair. Circ. Res. 2016, 118, 330–343. [Google Scholar] [CrossRef] [Green Version]
- Messina, E.; De Angelis, L.; Frati, G.; Morrone, S.; Chimenti, S.; Fiordaliso, F.; Salio, M.; Battaglia, M.; Latronico Michael, V.G.; Coletta, M.; et al. Isolation and Expansion of Adult Cardiac Stem Cells From Human and Murine Heart. Circ. Res. 2004, 95, 911–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barile, L.; Gherghiceanu, M.; Popescu, L.M.; Moccetti, T.; Vassalli, G. Human Cardiospheres as a Source of Multipotent Stem and Progenitor Cells. Stem Cells Int. 2013, 2013, 916837. [Google Scholar] [CrossRef] [PubMed]
- Ashur, C.; Frishman, W.H. Cardiosphere-Derived Cells and Ischemic Heart Failure. Cardiol. Rev. 2018, 26, 8–21. [Google Scholar] [CrossRef]
- Gallet, R.; Dawkins, J.; Valle, J.; Simsolo, E.; de Couto, G.; Middleton, R.; Tseliou, E.; Luthringer, D.; Kreke, M.; Smith, R.R.; et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 2017, 38, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.T.; Dawkins, J.; Bi, X.; Marbán, E.; Li, D. Diffusion Tensor Cardiac Magnetic Resonance Reveals Exosomes From Cardiosphere-Derived Cells Preserve Myocardial Fiber Architecture After Myocardial Infarction. JACC Basic Transl. Sci. 2018, 3, 97–109. [Google Scholar] [CrossRef]
- de Couto, G.; Gallet, R.; Cambier, L.; Jaghatspanyan, E.; Makkar, N.; Dawkins, J.F.; Berman, B.P.; Marbán, E. Exosomal MicroRNA Transfer Into Macrophages Mediates Cellular Postconditioning. Circulation 2017, 136, 200–214. [Google Scholar] [CrossRef] [PubMed]
- López, E.; Blázquez, R.; Marinaro, F.; Álvarez, V.; Blanco, V.; Báez, C.; González, I.; Abad, A.; Moreno, B.; Sánchez-Margallo, F.M.; et al. The Intrapericardial Delivery of Extracellular Vesicles from Cardiosphere-Derived Cells Stimulates M2 Polarization during the Acute Phase of Porcine Myocardial Infarction. Stem Cell Rev. Rep. 2020, 16, 612–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potz, B.A.; Scrimgeour, L.A.; Pavlov, V.I.; Sodha, N.R.; Abid, M.R.; Sellke, F.W. Extracellular Vesicle Injection Improves Myocardial Function and Increases Angiogenesis in a Swine Model of Chronic Ischemia. J. Am. Heart Assoc. 2018, 7, e008344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Chen, Y.; Zhao, Z.; Meng, Q.; Yu, Y.; Sun, J.; Yang, Z.; Chen, Y.; Li, J.; Ma, T.; et al. Engineered Exosomes With Ischemic Myocardium-Targeting Peptide for Targeted Therapy in Myocardial Infarction. J. Am. Heart Assoc. 2018, 7, e008737. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ding, N.; Guan, G.; Liu, G.; Huo, D.; Li, Y.; Wei, K.; Yang, J.; Cheng, P.; Zhu, C. Rapid Delivery of Hsa-miR-590-3p Using Targeted Exosomes to Treat Acute Myocardial Infarction Through Regulation of the Cell Cycle. J. Biomed. Nanotechnol. 2018, 14, 968–977. [Google Scholar] [CrossRef]
- Braga, L.; Ali, H.; Secco, I.; Giacca, M. Non-coding RNA therapeutics for cardiac regeneration. Cardiovasc. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gabisonia, K.; Prosdocimo, G.; Aquaro, G.D.; Carlucci, L.; Zentilin, L.; Secco, I.; Ali, H.; Braga, L.; Gorgodze, N.; Bernini, F.; et al. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature 2019, 569, 418–422. [Google Scholar] [CrossRef]
- Eulalio, A.; Mano, M.; Dal Ferro, M.; Zentilin, L.; Sinagra, G.; Zacchigna, S.; Giacca, M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature 2012, 492, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Krützfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature 2005, 438, 685–689. [Google Scholar] [CrossRef]
- Dirin, M.; Winkler, J. Influence of diverse chemical modifications on the ADME characteristics and toxicology of antisense oligonucleotides. Expert Opin. Biol. 2013, 13, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Elmén, J.; Lindow, M.; Schütz, S.; Lawrence, M.; Petri, A.; Obad, S.; Lindholm, M.; Hedtjärn, M.; Hansen, H.F.; Berger, U.; et al. LNA-mediated microRNA silencing in non-human primates. Nature 2008, 452, 896–899. [Google Scholar] [CrossRef]
- Ucar, A.; Gupta, S.K.; Fiedler, J.; Erikci, E.; Kardasinski, M.; Batkai, S.; Dangwal, S.; Kumarswamy, R.; Bang, C.; Holzmann, A.; et al. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat. Commun. 2012, 3, 1078. [Google Scholar] [CrossRef]
- Foinquinos, A.; Batkai, S.; Genschel, C.; Viereck, J.; Rump, S.; Gyöngyösi, M.; Traxler, D.; Riesenhuber, M.; Spannbauer, A.; Lukovic, D.; et al. Preclinical development of a miR-132 inhibitor for heart failure treatment. Nat. Commun. 2020, 11, 633. [Google Scholar] [CrossRef]
- Bonauer, A.; Carmona, G.; Iwasaki, M.; Mione, M.; Koyanagi, M.; Fischer, A.; Burchfield, J.; Fox, H.; Doebele, C.; Ohtani, K.; et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 2009, 324, 1710–1713. [Google Scholar] [CrossRef] [Green Version]
- Hinkel, R.; Penzkofer, D.; Zühlke, S.; Fischer, A.; Husada, W.; Xu, Q.F.; Baloch, E.; van Rooij, E.; Zeiher, A.M.; Kupatt, C.; et al. Inhibition of microRNA-92a protects against ischemia/reperfusion injury in a large-animal model. Circulation 2013, 128, 1066–1075. [Google Scholar] [CrossRef] [Green Version]
- Bellera, N.; Barba, I.; Rodriguez-Sinovas, A.; Ferret, E.; Asín, M.A.; Gonzalez-Alujas, M.T.; Pérez-Rodon, J.; Esteves, M.; Fonseca, C.; Toran, N.; et al. Single intracoronary injection of encapsulated antagomir-92a promotes angiogenesis and prevents adverse infarct remodeling. J. Am. Heart Assoc. 2014, 3, e000946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kormann, M.S.; Hasenpusch, G.; Aneja, M.K.; Nica, G.; Flemmer, A.W.; Herber-Jonat, S.; Huppmann, M.; Mays, L.E.; Illenyi, M.; Schams, A.; et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 2011, 29, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Zangi, L.; Lui, K.O.; von Gise, A.; Ma, Q.; Ebina, W.; Ptaszek, L.M.; Später, D.; Xu, H.; Tabebordbar, M.; Gorbatov, R.; et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 2013, 31, 898–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbull, I.C.; Eltoukhy, A.A.; Fish, K.M.; Nonnenmacher, M.; Ishikawa, K.; Chen, J.; Hajjar, R.J.; Anderson, D.G.; Costa, K.D. Myocardial Delivery of Lipidoid Nanoparticle Carrying modRNA Induces Rapid and Transient Expression. Mol. Ther. J. Am. Soc. Gene Ther. 2016, 24, 66–75. [Google Scholar] [CrossRef]
- Rogg, E.M.; Abplanalp, W.T.; Bischof, C.; John, D.; Schulz, M.H.; Krishnan, J.; Fischer, A.; Poluzzi, C.; Schaefer, L.; Bonauer, A.; et al. Analysis of Cell Type-Specific Effects of MicroRNA-92a Provides Novel Insights Into Target Regulation and Mechanism of Action. Circulation 2018, 138, 2545–2558. [Google Scholar] [CrossRef]
- Lesizza, P.; Prosdocimo, G.; Martinelli, V.; Sinagra, G.; Zacchigna, S.; Giacca, M. Single-Dose Intracardiac Injection of Pro-Regenerative MicroRNAs Improves Cardiac Function After Myocardial Infarction. Circ. Res. 2017, 120, 1298–1304. [Google Scholar] [CrossRef]
- Chamberlain, K.; Riyad, J.M.; Weber, T. Cardiac gene therapy with adeno-associated virus-based vectors. Curr. Opin. Cardiol. 2017. [Google Scholar] [CrossRef]
- Tao, Z.; Chen, B.; Tan, X.; Zhao, Y.; Wang, L.; Zhu, T.; Cao, K.; Yang, Z.; Kan, Y.W.; Su, H. Coexpression of VEGF and angiopoietin-1 promotes angiogenesis and cardiomyocyte proliferation reduces apoptosis in porcine myocardial infarction (MI) heart. Proc. Natl. Acad. Sci. USA 2011, 108, 2064–2069. [Google Scholar] [CrossRef] [Green Version]
- Kupatt, C.; Hinkel, R.; Pfosser, A.; El-Aouni, C.; Wuchrer, A.; Fritz, A.; Globisch, F.; Thormann, M.; Horstkotte, J.; Lebherz, C.; et al. Cotransfection of vascular endothelial growth factor-A and platelet-derived growth factor-B via recombinant adeno-associated virus resolves chronic ischemic malperfusion role of vessel maturation. J. Am. Coll. Cardiol. 2010, 56, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Bulysheva, A.A.; Hargrave, B.; Burcus, N.; Lundberg, C.G.; Murray, L.; Heller, R. Vascular endothelial growth factor-A gene electrotransfer promotes angiogenesis in a porcine model of cardiac ischemia. Gene Ther. 2016, 23, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Woitek, F.; Zentilin, L.; Hoffman, N.E.; Powers, J.C.; Ottiger, I.; Parikh, S.; Kulczycki, A.M.; Hurst, M.; Ring, N.; Wang, T.; et al. Intracoronary Cytoprotective Gene Therapy: A Study of VEGF-B167 in a Pre-Clinical Animal Model of Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2015, 66, 139–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, C.; Oduk, Y.; Zhao, M.; Lou, X.; Tang, Y.; Pretorius, D.; Valarmathi, M.T.; Walcott, G.P.; Yang, J.; Menasche, P.; et al. Myocardial protection by nanomaterials formulated with CHIR99021 and FGF1. JCI Insight 2020, 5, e132796. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.P.A.; Talman, V.; Torrieri, G.; Liu, D.; Marques, G.; Moslova, K.; Liu, Z.; Pinto, J.F.; Hirvonen, J.; Ruskoaho, H.; et al. Dual-Drug Delivery Using Dextran-Functionalized Nanoparticles Targeting Cardiac Fibroblasts for Cellular Reprogramming. Adv. Funct. Mater. 2018, 28, 1705134. [Google Scholar] [CrossRef]
- Báez-Díaz, C.; Blanco-Blázquez, V.; Sánchez-Margallo, F.M.; Bayes-Genis, A.; González, I.; Abad, A.; Steendam, R.; Franssen, O.; Palacios, I.; Sánchez, B.; et al. Microencapsulated Insulin-Like Growth Factor-1 therapy improves cardiac function and reduces fibrosis in a porcine acute myocardial infarction model. Sci. Rep. 2020, 10, 7166. [Google Scholar] [CrossRef] [PubMed]
- Thavapalachandran, S.; Grieve, S.M.; Hume, R.D.; Le, T.Y.L.; Raguram, K.; Hudson, J.E.; Pouliopoulos, J.; Figtree, G.A.; Dye, R.P.; Barry, A.M.; et al. Platelet-derived growth factor-AB improves scar mechanics and vascularity after myocardial infarction. Sci. Transl. Med. 2020, 12, eaay2140. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The Extracellular Matrix in Ischemic and Nonischemic Heart Failure. Circ. Res. 2019, 125, 117–146. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, M.L.; Zamilpa, R. Temporal and spatial expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases following myocardial infarction. Cardiovasc. Ther. 2012, 30, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Lindsey, M.L.; Gannon, J.; Aikawa, M.; Schoen, F.J.; Rabkin, E.; Lopresti-Morrow, L.; Crawford, J.; Black, S.; Libby, P.; Mitchell, P.G.; et al. Selective matrix metalloproteinase inhibition reduces left ventricular remodeling but does not inhibit angiogenesis after myocardial infarction. Circulation 2002, 105, 753–758. [Google Scholar] [CrossRef] [Green Version]
- Kandalam, V.; Basu, R.; Abraham, T.; Wang, X.; Awad, A.; Wang, W.; Lopaschuk, G.D.; Maeda, N.; Oudit, G.Y.; Kassiri, Z. Early activation of matrix metalloproteinases underlies the exacerbated systolic and diastolic dysfunction in mice lacking TIMP3 following myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1012–H1023. [Google Scholar] [CrossRef]
- Barlow, S.C.; Doviak, H.; Jacobs, J.; Freeburg, L.A.; Perreault, P.E.; Zellars, K.N.; Moreau, K.; Villacreses, C.F.; Smith, S.; Khakoo, A.Y.; et al. Intracoronary delivery of recombinant TIMP-3 after myocardial infarction: Effects on myocardial remodeling and function. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H690–H699. [Google Scholar] [CrossRef]
- Baehr, A.; Umansky, K.B.; Bassat, E.; Jurisch, V.; Klett, K.; Bozoglu, T.; Hornaschewitz, N.; Solyanik, O.; Kain, D.; Ferraro, B.; et al. Agrin Promotes Coordinated Therapeutic Processes Leading to Improved Cardiac Repair in Pigs. Circulation 2020. [Google Scholar] [CrossRef] [PubMed]
- Bassat, E.; Mutlak, Y.E.; Genzelinakh, A.; Shadrin, I.Y.; Baruch Umansky, K.; Yifa, O.; Kain, D.; Rajchman, D.; Leach, J.; Riabov Bassat, D.; et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 2017, 547, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Serpooshan, V.; Hurtado, C.; Diez-Cuñado, M.; Zhao, M.; Maruyama, S.; Zhu, W.; Fajardo, G.; Noseda, M.; Nakamura, K.; et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 2015, 525, 479–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karra, R.; Foglia, M.J.; Choi, W.Y.; Belliveau, C.; DeBenedittis, P.; Poss, K.D. Vegfaa instructs cardiac muscle hyperplasia in adult zebrafish. Proc. Natl. Acad. Sci. USA 2018, 115, 8805–8810. [Google Scholar] [CrossRef] [Green Version]
- Ferraro, B.; Leoni, G.; Hinkel, R.; Ormanns, S.; Paulin, N.; Ortega-Gomez, A.; Viola, J.R.; de Jong, R.; Bongiovanni, D.; Bozoglu, T.; et al. Pro-Angiogenic Macrophage Phenotype to Promote Myocardial Repair. J. Am. Coll. Cardiol. 2019, 73, 2990–3002. [Google Scholar] [CrossRef]
- Shapiro, S.D.; Ranjan, A.K.; Kawase, Y.; Cheng, R.K.; Kara, R.J.; Bhattacharya, R.; Guzman-Martinez, G.; Sanz, J.; Garcia, M.J.; Chaudhry, H.W. Cyclin A2 induces cardiac regeneration after myocardial infarction through cytokinesis of adult cardiomyocytes. Sci. Transl. Med. 2014, 6, 224ra227. [Google Scholar] [CrossRef]
- Ishikawa, K.; Fish, K.; Aguero, J.; Yaniz-Galende, E.; Jeong, D.; Kho, C.; Tilemann, L.; Fish, L.; Liang, L.; Eltoukhy, A.A.; et al. Stem cell factor gene transfer improves cardiac function after myocardial infarction in swine. Circ. Heart Fail. 2015, 8, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, T.; Kraus, M.; Husada, W.; Gesenhues, F.; Jiang, Q.; Pinkenburg, O.; Trenkwalder, T.; Laugwitz, K.-L.; le Noble, F.; Weber, C.; et al. Steerable Induction of the Thymosin β4/MRTF-A Pathway via AAV-Based Overexpression Induces Therapeutic Neovascularization. Hum. Gene Ther. 2017, 29, 1407–1415. [Google Scholar] [CrossRef]
- Facchin, F.; Canaider, S.; Tassinari, R.; Zannini, C.; Bianconi, E.; Taglioli, V.; Olivi, E.; Cavallini, C.; Tausel, M.; Ventura, C. Physical energies to the rescue of damaged tissues. World J. Stem Cells 2019, 11, 297–321. [Google Scholar] [CrossRef]
- Nishida, T.; Shimokawa, H.; Oi, K.; Tatewaki, H.; Uwatoku, T.; Abe, K.; Matsumoto, Y.; Kajihara, N.; Eto, M.; Matsuda, T.; et al. Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia-induced myocardial dysfunction in pigs in vivo. Circulation 2004, 110, 3055–3061. [Google Scholar] [CrossRef] [Green Version]
- Gollmann-Tepeköylü, C.; Lobenwein, D.; Theurl, M.; Primessnig, U.; Lener, D.; Kirchmair, E.; Mathes, W.; Graber, M.; Pölzl, L.; An, A.; et al. Shock Wave Therapy Improves Cardiac Function in a Model of Chronic Ischemic Heart Failure: Evidence for a Mechanism Involving VEGF Signaling and the Extracellular Matrix. J. Am. Heart Assoc. 2018, 7, e010025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimpfer, D.; Aharinejad, S.; Holfeld, J.; Thomas, A.; Dumfarth, J.; Rosenhek, R.; Czerny, M.; Schaden, W.; Gmeiner, M.; Wolner, E.; et al. Direct epicardial shock wave therapy improves ventricular function and induces angiogenesis in ischemic heart failure. J. Thorac. Cardiovasc. Surg. 2009, 137, 963–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holfeld, J.; Zimpfer, D.; Albrecht-Schgoer, K.; Stojadinovic, A.; Paulus, P.; Dumfarth, J.; Thomas, A.; Lobenwein, D.; Tepeköylü, C.; Rosenhek, R.; et al. Epicardial shock-wave therapy improves ventricular function in a porcine model of ischaemic heart disease. J. Tissue Eng. Regen. Med. 2016, 10, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Blatt, A.; Elbaz-Greener, G.A.; Tuby, H.; Maltz, L.; Siman-Tov, Y.; Ben-Aharon, G.; Copel, L.; Eisenberg, I.; Efrati, S.; Jonas, M.; et al. Low-Level Laser Therapy to the Bone Marrow Reduces Scarring and Improves Heart Function Post-Acute Myocardial Infarction in the Pig. Photomed. Laser Surg. 2016, 34, 516–524. [Google Scholar] [CrossRef]
- Oron, U.; Yaakobi, T.; Oron, A.; Hayam, G.; Gepstein, L.; Rubin, O.; Wolf, T.; Ben Haim, S. Attenuation of infarct size in rats and dogs after myocardial infarction by low-energy laser irradiation. Lasers Surg. Med. 2001, 28, 204–211. [Google Scholar] [CrossRef]
- Oron, U.; Yaakobi, T.; Oron, A.; Mordechovitz, D.; Shofti, R.; Hayam, G.; Dror, U.; Gepstein, L.; Wolf, T.; Haudenschild, C.; et al. Low-energy laser irradiation reduces formation of scar tissue after myocardial infarction in rats and dogs. Circulation 2001, 103, 296–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genau, M.C.; Perreault, P.E.; Romito, E.; Doviak, H.; Logdon, C.B.; Ruble, S.; Spinale, F.G. Institution of localized high-frequency electrical stimulation targeting early myocardial infarction: Effects on left ventricle function and geometry. J. Thorac. Cardiovasc. Surg. 2018, 156, 568–575. [Google Scholar] [CrossRef]
- Mahmoud, A.I.; O’Meara, C.C.; Gemberling, M.; Zhao, L.; Bryant, D.M.; Zheng, R.; Gannon, J.B.; Cai, L.; Choi, W.Y.; Egnaczyk, G.F.; et al. Nerves Regulate Cardiomyocyte Proliferation and Heart Regeneration. Dev. Cell 2015, 34, 387–399. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.Y.; Liu, Y.; Zuo, M.; Zhang, Y.; Yue, W.; Au, K.W.; Lai, W.H.; Wu, Y.; Shuto, C.; Chen, P.; et al. Remodelling of cardiac sympathetic re-innervation with thoracic spinal cord stimulation improves left ventricular function in a porcine model of heart failure. Europace 2015, 17, 1875–1883. [Google Scholar] [CrossRef]
- Hamann, J.J.; Ruble, S.B.; Stolen, C.; Wang, M.; Gupta, R.C.; Rastogi, S.; Sabbah, H.N. Vagus nerve stimulation improves left ventricular function in a canine model of chronic heart failure. Eur. J. Heart Fail. 2013, 15, 1319–1326. [Google Scholar] [CrossRef] [Green Version]
- Brandt, E.B.; Bashar, S.J.; Mahmoud, A.I. Stimulating ideas for heart regeneration: The future of nerve-directed heart therapy. Bioelectron. Med. 2019, 5, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghiroldi, A.; Piccoli, M.; Ciconte, G.; Pappone, C.; Anastasia, L. Regenerating the human heart: Direct reprogramming strategies and their current limitations. Basic Res. Cardiol. 2017, 112, 68. [Google Scholar] [CrossRef] [PubMed]
- Ieda, M.; Fu, J.D.; Delgado-Olguin, P.; Vedantham, V.; Hayashi, Y.; Bruneau, B.G.; Srivastava, D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010, 142, 375–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inagawa, K.; Miyamoto, K.; Yamakawa, H.; Muraoka, N.; Sadahiro, T.; Umei, T.; Wada, R.; Katsumata, Y.; Kaneda, R.; Nakade, K.; et al. Induction of cardiomyocyte-like cells in infarct hearts by gene transfer of Gata4, Mef2c, and Tbx5. Circ. Res. 2012, 111, 1147–1156. [Google Scholar] [CrossRef]
- Qian, L.; Huang, Y.; Spencer, C.I.; Foley, A.; Vedantham, V.; Liu, L.; Conway, S.J.; Fu, J.D.; Srivastava, D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 2012, 485, 593–598. [Google Scholar] [CrossRef]
- Song, K.; Nam, Y.J.; Luo, X.; Qi, X.; Tan, W.; Huang, G.N.; Acharya, A.; Smith, C.L.; Tallquist, M.D.; Neilson, E.G.; et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature 2012, 485, 599–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayawardena, T.M.; Egemnazarov, B.; Finch, E.A.; Zhang, L.; Payne, J.A.; Pandya, K.; Zhang, Z.; Rosenberg, P.; Mirotsou, M.; Dzau, V.J. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ. Res. 2012, 110, 1465–1473. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Huang, C.; Xu, X.; Gu, H.; Ye, Y.; Jiang, C.; Qiu, Z.; Xie, X. Direct reprogramming of mouse fibroblasts into cardiomyocytes with chemical cocktails. Cell Res. 2015, 25, 1013–1024. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.P.; Mathison, M.; Patel, V.; Sanagasetti, D.; Gibson, B.W.; Yang, J.; Rosengart, T.K. MiR-590 Promotes Transdifferentiation of Porcine and Human Fibroblasts Toward a Cardiomyocyte-Like Fate by Directly Repressing Specificity Protein 1. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef]
- Palazzolo, G.; Quattrocelli, M.; Toelen, J.; Dominici, R.; Anastasia, L.; Tettamenti, G.; Barthelemy, I.; Blot, S.; Gijsbers, R.; Cassano, M.; et al. Cardiac Niche Influences the Direct Reprogramming of Canine Fibroblasts into Cardiomyocyte-Like Cells. Stem Cells Int. 2016, 2016, 4969430. [Google Scholar] [CrossRef] [Green Version]
- Paoletti, C.; Divieto, C.; Tarricone, G.; Di Meglio, F.; Nurzynska, D.; Chiono, V. MicroRNA-Mediated Direct Reprogramming of Human Adult Fibroblasts Toward Cardiac Phenotype. Front. Bioeng. Biotechnol. 2020, 8, 529. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Huang, Y.; Zheng, J.; Spencer, C.I.; Zhang, Y.; Fu, J.D.; Nie, B.; Xie, M.; Zhang, M.; Wang, H.; et al. Conversion of human fibroblasts into functional cardiomyocytes by small molecules. Science 2016, 352, 1216–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Stem Cell Secretomes and EVs. | ||||
|---|---|---|---|---|
| Therapy Delivery Method | Animal Model Sample Size Follow-Up | Main Effects | Proposed Mechanism | |
| MSC-CM i.v. + i.c. | Pig (AMI) (n = 30) 4 h | ↑ LVEF (+32%) ↓ Infarct size (−60%) | ↓ TGF-β signaling ↓ Apoptosis | [24] |
| MSC-CM i.v. | Pig (AMI) (n = 22) 3 w | ↑ LVEF (+37%) ↑ Capillary density (+50%) | [25] | |
| EPC-CM i.c. | Pig (AMI) (n = 56) 24 h/8 w | ↑ ± dp/dt ↓ Infarct size (−37%) | ↑ IGF-1 signaling | [26] |
| APOSEC m.c. | Pig (AMI) (n = 16) 60 d | ↑ Cardiac index ↓ Infarct size (−42%) | ↑ Angiogenesis ↓ Apoptosis | [27,28] |
| CDCexo i.c./m.c. | Pig (AMI) (n = 22) 48 h (n = 13) 60 d | ↑ LVEF (+21.27%) 48 h ↓ Infarct size (−26.9%) 48 h ↑ LVEF (+13.3%) 60 d ↓ ESV (−17.47%) 60 d | ↑ Anti-inflammatory macrophage polarization ↓ Fibrosis ↑ Angiogenesis | [34,35] |
| CDCexo m.c. | Pig (AMI) (n = 9) 48 h | ↑ LVEF (+18%) ↓ MVO ↓ CD68+ macrophages | [36] | |
| CDCexo intrapericardial | Pig (AMI) (n = 18) 10 w | ↑ M2 macrophages | [22,37] | |
| MSCexom.c. | Pig (ameroid constrictor)(n = 23)7 w | ↑ Stroke volume (+33.7%)↑ Capillary density | ↑ Angiogenesis | [38] |
| RNA-Based Therapies | ||||
|---|---|---|---|---|
| Molecule (Delivery Method) | Animal Model Sample Size Follow-Up | Main Effects | Proposed Mechanism | |
| miR-199a AAV6 mc. | Pig (AMI) (n = 19) 8 w | ↑ LVEF (+17.1%) ↓ Scar size/mass (−50%) ↓ Fibrosis | CM cell-cycle reentry | [42] |
| antimiR-132 i.c./i.v. | Pig (AMI) (n = 156) 56 d | ↑ LVEF ↓ Scar size ↓ Fibrosis ↓ CM size | ↑ Foxo3 (anti-fibrotic)↑ Serca2a | [48] |
| antagomiR-92a i.v./i.c. | Pig (AMI) (n = 30) 7 d | ↑ LVEF ↑ Capillary density | ↑ Angiogenesis ↓ Inflammation ↓ CM apoptosis | [50] |
| PLGA antagomir-92a i.c. | Pig (AMI) (n = 27) 10 d | ↑ LVEF ↓ Adverse remodeling | [51] | |
| Growth Factors, Proteins, and Other Molecules | ||||
|---|---|---|---|---|
| Molecule Delivery Method | Animal Model Sample Size Follow-Up | Main Effects | Proposed Mechanism | |
| VEGF + angiopoietin-1 AAV mc. | Pig (AMI) (n = 24) 8 w | ↑ LVEF ↑ Capillary density | ↑ Angiogenesis ↑ CM proliferation ↓ Apoptosis | [58] |
| VEGF + PDGF-B AAV i.c. | Pig (reduction stent) (n = 27) 56 d | ↑ LVEF ↑ Collateralization | [59] | |
| VEGF-A plasmid + GET mc. | Pig (AMI) (n = 37) 7 w | ↑ Angiogenesis | [60] | |
| VEGF-B167 AAV i.c. | Canine (dilated CMP) (n = 53) | ↑ LVEF ↓ LVEDP ↑ dP/dtmax | ↓ Apoptosis | [61] |
| FGF-1 + CHIR99021 NPs mc. | Pig (AMI) (n = 12) 28 d | ↑ LVEF ↑ Angiogenesis | CM cell cycle reentry | [62] |
| Microencapsulated IGF-1 i.c. | Pig (AMI) (n = 24) 10 w | ↑ LVEF (+18%) ↓ CVF ↓ ESVi | ↑ Angiogenesis ↓ Apoptosis | [64] |
| rhPDGF-AB i.v. | Pig (AMI) (n = 36) 28 d | ↑ Survival ↑ LVEF ↑ Scar anisotropy ↓ VT | ↑ Angiogenesis Fibroblast modulation | [65] |
| TIMP-3 i.c. | Pig (AMI) (n = 17) 28 d | ↓ Infarct size (−45%) ↓ LV dilation (−40%) | MMP inhibition | [70] |
| rhAgrin i.c. | Pig (AMI) (n = 19) 28 d | ↑ LVEF ↓ Scar size ↓ Adverse remodeling | CM cell-cycle reentry ↑ Angiogenesis ↓ Inflammation | [71] |
| Fstl-1 Epicardial patch | Pig (AMI) (n = 6) 5 w | ↑ LVEF ↓ Scar size ↑ CM proliferation ↑ Arteriogenesis | ↑ Cardiogenesis ↓ Apoptosis | [73] |
| AnxA1 cardiotropic AAV i.v. | Pig (AMI) (n = 7) 7 d | ↑ VEGF-A Macrophage polarization | Macrophage modulation | [75] |
| Ccna2 AAV mc. | Pig (AMI) (n = 27) 6 w | ↑ LVEF ↑ CM proliferation | CM cell-cycle reentry | [76] |
| SCF mc. | Pig (AMI) (n = 22) 3 m | ↑ LVEF (+12%) ↑ Angiogenesis | ↑ Angiogenesis ↓ Apoptosis | [77] |
| Tβ4 + MRTF-A AAV i.v. | Pig (reduction stent) (n = 20) 56 d | ↑ LVEF ↓ LVEDP ↑ Collateralization | ↓ Apoptosis ↑ Collateralization | [78] |
| Physical Stimulation | ||||
|---|---|---|---|---|
| Therapy Delivery Method Target Area | Animal Model Sample Size Follow-Up | Main Effects | Proposed Mechanism | |
| SWT Extracorporeal Ischemic myocardium | Pig (Ameroid constrictor) (n = 16) 8 w | ↑ LVEF ↑ WTF ↑ Angiogenesis | ↑ VEGF | [80] |
| SWT Invasive Ischemic myocardium | Pig (AMI) (n = 11) 6 w | ↑ LVEF ↑ Angiogenesis | [83] | |
| LLLT Invasive Bone marrow | Pig (AMI) (n = 12) 90 d | ↓ Infarct size (−68%) ↑ Angiogenesis | ↑ VEGF ↑ Stem cell proliferation | [84] |
| LLLT Invasive Ischemic myocardium | Canine (AMI) (n = 22) 14 d | ↓ Infarct size (−49%) | [85] | |
| LLLT Invasive Ischemic myocardium | Canine (AMI) (n = 50) 6 w | ↓ Infarct size (−52%) | [86] | |
| LHFS Invasive Ischemic myocardium | Pig (AMI) (n = 11) 28 d | EDV (32% vs. 12%) PCWP (+62% vs. −17%) | ↓ Adverse remodeling | [87] |
| SCS Intermittent/continuous T1-T3 | Pig (AMI + Pacing) (n = 30) 10 w | ↑ LVEF ↑ +dP/dt | ↑ Sympathetic nerve sprouting | [89] |
| VNS Implanted Electrode Vagus nerve | Canine (Microembolization) (n = 26) 6 m | ↑ LVEF ↓ ESV | ↓ Inflammation Macrophage modulation | [90] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spannbauer, A.; Mester-Tonczar, J.; Traxler, D.; Kastner, N.; Zlabinger, K.; Hašimbegović, E.; Riesenhuber, M.; Pavo, N.; Goliasch, G.; Gyöngyösi, M. Large Animal Models of Cell-Free Cardiac Regeneration. Biomolecules 2020, 10, 1392. https://doi.org/10.3390/biom10101392
Spannbauer A, Mester-Tonczar J, Traxler D, Kastner N, Zlabinger K, Hašimbegović E, Riesenhuber M, Pavo N, Goliasch G, Gyöngyösi M. Large Animal Models of Cell-Free Cardiac Regeneration. Biomolecules. 2020; 10(10):1392. https://doi.org/10.3390/biom10101392
Chicago/Turabian StyleSpannbauer, Andreas, Julia Mester-Tonczar, Denise Traxler, Nina Kastner, Katrin Zlabinger, Ena Hašimbegović, Martin Riesenhuber, Noemi Pavo, Georg Goliasch, and Mariann Gyöngyösi. 2020. "Large Animal Models of Cell-Free Cardiac Regeneration" Biomolecules 10, no. 10: 1392. https://doi.org/10.3390/biom10101392