Recovery of Bioactive Compounds from Hazelnuts and Walnuts Shells: Quantitative–Qualitative Analysis and Chromatographic Purification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation
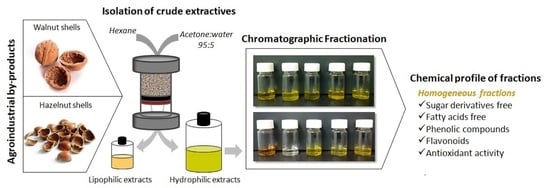
2.2. Crude Extracts from Shells
2.3. Analysis of Raw Extracts
2.4. Fractionation and Characterization of the Hydrophilic Extracts
2.5. Preliminary Tests of TPC and Antioxidant Activity of the Hydrophilic Fractions
2.6. Statistical Analysis
3. Results and Discussion
3.1. Extracts Yields and Chemical Composition
3.2. Isolation of Component Groups from Hydrophilic Fractions
3.3. TPC and Antioxidant Activity of Isolated Fractions from Crude Hydrophilic Extracts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gaspar, M.C.; Mendes, C.V.T.; Pinela, S.R.; Moreira, R.; Carvalho, M.G.V.S.; Quina, M.J.; Braga, M.E.M.; Portugal, A.T. Assessment of Agroforestry Residues: Their Potential within the Biorefinery Context. ACS Sustain. Chem. Eng. 2019, 7, 17154–17165. [Google Scholar] [CrossRef]
- Andrea, T.; Lars, W.; Dennis, S.; Axel, T. Assessment of agroforestry residue potentials for the bioeconomy inthe European Union. J. Clean. Prod. 2018, 176, 348–359. [Google Scholar]
- Gregg, J.S.; Jürgens, J.; Happel, M.K.; Strøm-Andersen, N.; Tanner, A.N.; Bolwig, S.; Klitkou, A. Valorization of bio-residuals in the food and forestry sectors in support of a circular bioeconomy: A review. J. Clean. Prod. 2017, 144, 559–571. [Google Scholar] [CrossRef]
- Khir, R.; Pan, Z. Walnuts. In Integrated Processing Technologies for Food and Agricultural By-Products; Academic Press: Cambridge, MA, USA, 2019; ISBN 9780128141397. [Google Scholar]
- Contini, M.; Baccelloni, S.; Massantini, R.; Anelli, G. Extraction of natural antioxidants from hazelnut (Corylus avellana L.) shell and skin wastes by long maceration at room temperature. Food Chem. 2008, 110, 659–669. [Google Scholar] [CrossRef]
- FAOSTAT. World Food and Agriculture 2018: Statistical Pocketbook; FAO: Rome, Italy, 2018; ISBN 9789251310120. [Google Scholar]
- Demirbaş, A. Estimating of structural composition of wood and non-wood biomass samples. Energy Sources 2005, 27, 761–767. [Google Scholar] [CrossRef]
- Shah, M.A.; Khan, M.N.S.; Kumar, V. Biomass residue characterization for their potential application as biofuels. J. Therm. Anal. Calorim. 2018, 134, 2137–2145. [Google Scholar] [CrossRef]
- Ahorsu, R.; Cintorrino, G.; Medina, F.; Constantí, M. Microwave processes: A viable technology for obtaining xylose from walnut shell to produce lactic acid by Bacillus coagulans. J. Clean. Prod. 2019, 05, 289. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Ye, Z.Q.; Zhang, F.; Han, J.J.; Yang, T.; Wang, Z.Z.; Zhang, Y. Nutshell Extracts of Xanthoceras sorbifolia: A New Potential Source of Bioactive Phenolic Compounds as a Natural Antioxidant and Immunomodulator. J. Agric. Food Chem. 2018, 66, 3783–3792. [Google Scholar] [CrossRef]
- Hasan, M.H.; Adzib, M.S.M.; Ilham, Z. Potential antioxidants from crude extracts of roselle seeds and cashew nut shells for biodiesel storage stability improvement. In Proceedings of the AIP Conference, 29–30 April 2019; American Institute of Physics Inc.: Bandung, Indonesia, 2019; Volume 2129, p. 020087. [Google Scholar]
- Khan, M.K.; Paniwnyk, L.; Hassan, S. Polyphenols as Natural Antioxidants: Sources, Extraction and Applications in Food, Cosmetics and Drugs; Springer: Singapore, 2019; pp. 197–235. [Google Scholar]
- Kamala Kumari, P.V.; Akhila, S.; Srinivasa Rao, Y.; Rama Devi, B. Alternative to artificial preservatives. Syst. Rev. Pharm. 2019, 10, 99–102. [Google Scholar]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant activity of spices and their impact on human health: A review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef] [Green Version]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.-Y.O.; Lima, G.P.P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability. In Phenolic Compounds–Biological Activity; IntechOpen Limited: London, UK, 2017. [Google Scholar]
- Gill, B.S.; Navgeet; Qiu, F. Technologies for extraction and production of bioactive compounds. In Biotechnological Production of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780444643230. [Google Scholar]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chinese Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Zhang, Y.; Rosenberg, J.N.; Sharif, N.; Betenbaugh, M.J.; Wang, F. Efficient lipid extraction and quantification of fatty acids from algal biomass using accelerated solvent extraction (ASE). RSC Adv. 2016, 6, 29127–29134. [Google Scholar] [CrossRef]
- Sun, H.; Ge, X.; Lv, Y.; Wang, A. Application of accelerated solvent extraction in the analysis of organic contaminants, bioactive and nutritional compounds in food and feed. J. Chromatogr. A 2012, 1237, 1–23. [Google Scholar] [CrossRef]
- Willför, S.M.; Leppänen, A.-S.; Willför, S.; Hemming, J. Analysis of Extractives in Different Pulps-Method Development, Evaluation, and Recommendations; Abo Akademi University: Turku, Finland, 2006. [Google Scholar]
- Conde, E.; Fang, W.; Hemming, J.; Willför, S.; Domínguez, H.; Parajó, J.C. Recovery of bioactive compounds from Pinus pinaster wood by consecutive extraction stages. Wood Sci. Technol. 2014, 48, 311–323. [Google Scholar] [CrossRef]
- Willför, S.; Hemming, J.; Reunanen, M.; Holmbom, B. Phenolic and lipophilic extractives in Scots pine knots and stemwood. Holzforschung 2003, 57, 359–372. [Google Scholar] [CrossRef]
- Willför, S.M.; Smeds, A.I.; Holmbom, B.R. Chromatographic analysis of lignans. J. Chromatogr. A 2006, 1112, 64–77. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry of total phenolics with acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Gordobil, O.; Herrera, R.; Yahyaoui, M.; Ilk, S.; Kaya, M.; Labidi, J. Potential use of kraft and organosolv lignins as a natural additive for healthcare products. RSC Adv. 2018, 8, 24525–24533. [Google Scholar] [CrossRef] [Green Version]
- Biermann’s Handbook of Pulp and Paper; Elsevier: Amsterdam, The Netherlands, 2018. [CrossRef]
- Penton, Z.E. Chapter 10 Headspace gas chromatography. Compr. Anal. Chem. 2002, 37, 279–296. [Google Scholar] [CrossRef]
- Nisula, L. Linda Nisula Wood Extractives in Conifers A Study of Stemwood and Knots of Industrially Important Species; Åbo Akademi University: Åbo, Finland, 2018. [Google Scholar]
- Queirós, C.S.G.P.; Cardoso, S.; Lourenço, A.; Ferreira, J.; Miranda, I.; Lourenço, M.J.V.; Pereira, H. Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition. Biomass Convers. Biorefinery 2020, 10.1, 175–188. [Google Scholar] [CrossRef]
- Benouadah, N.; Pranovich, A.; Aliouche, D.; Hemming, J.; Smeds, A.; Willför, S. Analysis of extractives from Pinus halepensis and Eucalyptus camaldulensis as predominant trees in Algeria. Holzforschung 2018, 72, 7–104. [Google Scholar] [CrossRef]
- Smeds, A.I.; Vähäsalo, L.; Rahkila, J.; Eklund, P.C.; Willför, S.M. Chemical characterisation of polymerised extractives in bleached birch kraft pulp. Holzforschung 2019, 73, 1017–1033. [Google Scholar] [CrossRef]
- Benouadah, N.; Aliouche, D.; Pranovich, A.; Willför, S. Chemical characterization of Pinus halepensis sapwood and heartwood. Wood Mater. Sci. Eng. 2019, 14, 157–164. [Google Scholar] [CrossRef]
- Kim, H.J.; Oh, M.S.; Hong, J.; Jang, Y.P. Quantitative analysis of major dibenzocyclooctane lignans in schisandrae fructus by online TLC-DART-MS. Phytochem. Anal. 2011, 22, 258–262. [Google Scholar] [CrossRef]
- Pérez-Armada, L.; Rivas, S.; González, B.; Moure, A. Extraction of phenolic compounds from hazelnut shells by green processes. J. Food Eng. 2019, 255, 1–8. [Google Scholar] [CrossRef]
- Esposito, T.; Sansone, F.; Franceschelli, S.; Del Gaudio, P.; Picerno, P.; Aquino, R.P.; Mencherini, T. Hazelnut (Corylus avellana L.) shells extract: Phenolic composition, antioxidant effect and cytotoxic activity on human cancer cell lines. Int. J. Mol. Sci. 2017, 18, 392. [Google Scholar] [CrossRef]
- Yuan, B.; Lu, M.; Eskridge, K.M.; Isom, L.D.; Hanna, M.A. Extraction, identification, and quantification of antioxidant phenolics from hazelnut (Corylus avellana L.) shells. Food Chem. 2018, 244, 7–15. [Google Scholar] [CrossRef]
- Squillaci, G.; Apone, F.; Sena, L.M.; Carola, A.; Tito, A.; Bimonte, M.; Lucia, A.D.E.; Colucci, G.; Cara, F.L.A.; Morana, A. Chestnut (Castanea sativa Mill.) industrial wastes as a valued bioresource for the production of active ingredients. Process. Biochem. 2018, 64, 228–236. [Google Scholar] [CrossRef]
- Ghasemi, K.; Ghasemi, Y.; Ehteshamnia, A.; Nabavi, S.M.; Nabavi, S.F.; Ebrahimzadeh, M.A.; Pourmorad, F. Influence of environmental factors on antioxidant activity, phenol and flavonoids contents of walnut (Juglans regia L.) green husks. J. Med. Plants Res. 2011, 5, 1128–1133. [Google Scholar]
- Amir JALILI Reducing power and radical scavenging activities of phenolic extracts from Juglans regia hulls and shells. AFRICAN J. Biotechnol. 2012, 11, 9040–9047. [CrossRef]
- Holmbom, B.; Willfoer, S.; Hemming, J.; Pietarinen, S.; Nisula, L.; Eklund, P.; Sjoeholm, R. Knots in trees: A rich source of bioactive polyphenols. In Proceedings of the ACS Symposium Series; Oxford University Press: Oxford, UK, 2007; Volume 964, pp. 350–362. [Google Scholar]
- Smeds, A.I.; Eklund, P.C.; Willför, S.M. Content, composition, and stereochemical characterisation of lignans in berries and seeds. Food Chem. 2012, 134, 1991–1998. [Google Scholar] [CrossRef] [PubMed]




| Sample | Extracted Groups | Gravimetric Quantification (mg/g) * | GC Quantification (mg/g) * |
|---|---|---|---|
| Hazelnuts shells | Lipophilic (L) | 9.64 ± 0.43 | 7.01 |
| Hydrophilic (H) | 13.80 ± 0.74 | 7.88 | |
| Total | 23.44 | 14.89 | |
| L/H ratio | 0.70 | 0.89 | |
| Walnuts shells | Lipophilic (L) | 5.24 ± 0.18 | 3.84 |
| Hydrophilic (H) | 22.68 ± 1.37 | 17.80 | |
| Total | 27.92 | 21.64 | |
| L/H ratio | 0.23 | 0.21 |
| Component Groups | Hazelnut Shell (mg/g) | Walnut Shell (mg/g) | |||||
|---|---|---|---|---|---|---|---|
| Lipophilic | Hydrophilic | Total | Lipophilic | Hydrophilic | Total | ||
| Fatty acids and alcohols | Unsaturated | 2.58 | 0.60 | 3.18 | 1.21 | 0.66 | 1.87 |
| Saturated | 0.45 | 0.03 | 0.48 | 0.35 | 0.29 | 0.64 | |
| Fatty alcohols | 0.01 | - | 0.01 | 0.02 | 0.40 | 0.42 | |
| Subtotal | 3.04 | 0.63 | 3.67 | 1.58 | 1.35 | 2.93 | |
| Terpenes | Terpenoids | 0.03 | 0.02 | 0.05 | 0.01 | 0.06 | 0.07 |
| Resin acids | Resin acids | 0.03 | 0.16 | 0.19 | 0.01 | 0.12 | 0.13 |
| Modified r.a. | - | 2.60 | 2.60 | - | 1.42 | 1.42 | |
| Subtotal | 0.06 | 2.78 | 2.84 | 0.02 | 1.60 | 1.62 | |
| Diglycerides | 0.26 | 1.05 | 1.31 | 0.23 | 0.45 | 0.68 | |
| Glycerides | Triglycerides | 3.13 | 0.33 | 3.46 | 1.40 | 0.13 | 1.53 |
| Subtotal | 3.39 | 1.38 | 4.77 | 1.63 | 0.58 | 2.21 | |
| Steroids | Sterols | 0.34 | 0.10 | 0.44 | 0.45 | 0.17 | 0.62 |
| Steryl esters | 0.10 | 0.17 | 0.27 | 0.10 | - | 0.10 | |
| Subtotal | 0.44 | 0.27 | 0.71 | 0.55 | 0.17 | 0.72 | |
| Phenolic compounds | Phenols, phenolic acids and lignin units | - | 0.64 | 0.64 | - | 2.22 | 2.22 |
| Lignans | - | 0.14 | 0.14 | - | 0.30 | 0.30 | |
| Stilbenes | 0.01 | 0.02 | |||||
| Flavonoids | - | 0.09 | 0.09 | - | 0.69 | 0.69 | |
| Unknown moieties | - | 0.03 | 0.03 | - | 0.23 | 0.23 | |
| Subtotal | - | 0.90 | 0.90 | - | 3.49 | 3.49 | |
| Other compounds | Sugars and derivatives | 0.66 | 0.66 | 0.01 | 9.21 | 9.22 | |
| Others | 0.03 | 0.62 | 0.65 | 0.02 | 1.27 | 1.29 | |
| Subtotal | 0.03 | 1.28 | 1.31 | 0.03 | 10.48 | 10.51 | |
| Non-identified | 0.05 | 0.64 | 0.69 | 0.03 | 0.13 | 0.16 | |
| TOTAL | 7.01 | 7.88 | 14.89 | 3.84 | 17.80 | 21.64 | |
| Compounds in Hazelnut Shell | Extract * | Fractions DCM-EtOH (mg/g) | |||||
|---|---|---|---|---|---|---|---|
| Fatty Acids and Alcohols | 97–3 (1) * | 97–3 (2) * | 97–3 (3) * | 96–4 * | 95–5 * | ||
| Fatty acids | Acid 9:0 | 0.01 a | - | - | - | - | - |
| Acid 18:0 | 0.02 a | 0.21 ± 0.02 | - | - | - | - | |
| Acid 18:1 | 0.45 ± 0.03 | - | 0.04 a | - | - | - | |
| Acid 18:2 | 0.09 ± 0.01 | 1.03 ± 0.14 | 0.01a | - | - | - | |
| Terpenes and Terpenoids | |||||||
| p-menthane-1,8-diol | 0.02 a | - | - | - | - | - | |
| Di-and triglycerides | |||||||
| Diglycerides | 1.05 ± 0.08 | 0.25 ± 0.01 | - | - | 0.02 a | 0.04 a | |
| Triglycerides | 0.33 ± 0.03 | - | - | - | 0.01a | - | |
| Resin Acids | |||||||
| Resin acids | Dehydroabietic acid | 0.02 a | - | - | - | - | - |
| Pimaric acid | - | 0.03 a | - | - | - | - | |
| Unidentified RT = 21:59–27:75 | 0.14 a | 0.03a | 0.03 a | 0.01 a | - | - | |
| Modified resin acids | 7-OH-dehydroabietic (DHA) acid | 0.47 ± 0.04 | - | - | - | - | - |
| X-OH-7oxoDHA acid | - | 0.03 a | - | 0.01 a | - | - | |
| Dihydroxy-DHA acid | 2.12 ± 0.21 | - | - | - | - | - | |
| 9-hydroxystearic acid | 0.01 a | 0.05 a | - | - | - | - | |
| Steroids | |||||||
| Sitosterol | 0.10 a | 0.81 ± 0.06 | 0.02 a | - | - | - | |
| Steryl esters | 0.17 ± 0.01 | 0.01a | 0.01 a | - | - | 0.02 a | |
| Phenolic Compounds | |||||||
| Phenols | Vanillin | 0.11 a | 0.38 ± 0.03 | - | - | - | - |
| 3,4-dihydroxybenzaldehyde | 0.02 a | - | - | - | - | - | |
| 3-methoxy-4-OH-cinnamaldehyde | 0.03 a | 0.19 a | - | - | - | - | |
| Gallic acid | 0.01 a | 0.78 ± 0.07 | 0.02 a | - | - | - | |
| Protocatechuic acid | 0.04 a | - | - | - | - | - | |
| Dihydroconiferyl alcohol | 0.01 a | 0.02a | - | - | - | - | |
| Vanillic acid | 0.07 a | - | - | 0.03 a | 0.03 a | - | |
| Phenolic acid | 4-hydroxybenzoic acid | 0.01 a | - | - | - | - | - |
| 1-guaiacyl-3-OH-1-propanone | 0.04 a | 0.01 a | 0.07 a | 0.04 a | - | - | |
| 1-guaiacyl-2-OH-1-ethanone | 0.01 a | 0.02 a | - | - | - | - | |
| 1-syringylglycerol | - | - | - | - | 0.02 a | - | |
| 1-guaiacyl -2,3-diOH-1-propanone | 0.05 a | 0.01 a | - | - | 0.07 a | - | |
| 1-guaiacylglycerol | 0.07 a | - | - | - | - | - | |
| 1,3-(bis-guaiacyl)-1,2-propandiol | 0.08 a | 0.01 a | - | - | 0.07 a | - | |
| P-hydroxyphenyl glycerol | - | - | - | - | 0.01 a | - | |
| Coniferyl alcohol | 0.07 a | 0.18 ± 0.02 | 0.01 a | - | - | - | |
| Monomethyl pinosylvin | 0.01 a | 0.01 a | - | - | - | - | |
| 4,4′-diOH-3,3′-dimethoxystilbene | 0.01 a | - | - | - | - | - | |
| Stilbenes and diarylheptanoids | pinosylvin | 0.01 a | - | - | - | - | - |
| X-c4h4(oh)3; c5h6(oh)3 | 0.03 a | 0.03 a | 0.01 a | - | 0.01 a | - | |
| Lignans | Olivil | 0.01 a | 0.04 a | - | - | - | - |
| glucopyranoside2[-4(OH)phe] | 0.01 a | - | - | - | - | - | |
| HMR | - | - | - | 0.08 a | - | - | |
| Todolactol | 0.12 ± 0.02 | - | - | 0.08 a | - | 0.05 a | |
| Flavonoids | Pinobanksin | 0.05 a | - | - | - | - | 0.05 a |
| (+)-Catechin | 0.01 a | 0.08 a | - | - | 0.01 a | 0.02 a | |
| Quercetin | 0.02 a | 0.12 ± 0.01 | 0.10 ± 0.01 | - | - | - | |
| Gallocatechin (1) | 0.01 a | - | - | - | - | - | |
| Unidentified RT = 27.09–27:80 | - | 0.01 a | - | - | - | 0.02 a | |
| Other Compounds | |||||||
| Sugar derivatives | Monosaccharides | 0.60 ± 0.08 | 0.40 ± 0.05 | - | - | - | 0.03 a |
| Sugar acids | 0.02 a | - | - | - | - | - | |
| Sugar alcohol | 0.02 a | - | - | - | - | - | |
| Others | Carboxyl acid | 0.58 ± 0.06 | - | - | - | - | - |
| Alpha-lapachone | 0.02 a | - | - | - | - | - | |
| Dehydro-alpha-lapachone | 0.01 a | - | - | - | - | - | |
| Maltol | 0.01 a | - | - | - | - | - | |
| Non-identified | 0.64 ± 0.11 | 0.15 ± 0.04 | - | - | - | - | |
| Compounds in Walnut Shell | Extract * | Fractions DCM-EtOH | |||||
|---|---|---|---|---|---|---|---|
| Fatty Acids and Alcohols | 97–3 (1) * | 97–3 (2) * | 97–3 (3) * | 96–4 * | 95–5 * | ||
| Fatty acids | Acid 9:0 | 0.25 ± 0.02 | - | - | - | - | - |
| Acid 18:0 | 0.04 a | - | 0.13 a | - | 0.01 a | - | |
| Acid 18:1 | 0.15 a | - | 0.56 ± 0.06 | - | - | - | |
| Acid 18:2 | 0.51 ± 0.05 | - | - | - | - | - | |
| Fatty alcohols | Alcohol 16:0 | 0.40 ± 0.02 | - | - | - | - | - |
| Terpenes and Terpenoids | |||||||
| Terpineol | - | - | 0.02 a | - | - | - | |
| p-menthane-1,8-diol | 0.06 a | - | - | - | - | - | |
| Di-and triglycerides | |||||||
| Diglycerides | 0.45 ± 0.04 | - | 0.06 a | - | - | 0.01 a | |
| Triglycerides | 0.13 a | - | - | - | - | 0.01 a | |
| Resin Acids | |||||||
| Resin acids | Isopimaric acid | 0.01a | - | - | - | - | - |
| Unidentified RT = 22:03-27:80 | 0.11 a | 0.02 a | 0.02 a | 0.02 a | - | - | |
| Modified resin acids | X-OH-7oxoDHA acid | - | - | 0.03 a | 0.01 a | - | - |
| Dihydroxy-DHA acid | 1.39 ± 0.21 | - | - | - | - | - | |
| 9-hydroxystearic acid | 0.01 a | - | 0.01 a | - | - | - | |
| X-hydroxyabietic acid | 0.02 a | - | - | - | - | - | |
| Steroids | |||||||
| Sitosterol | 0.17 ± 0.03 | - | 0.59 ± 0.09 | - | - | - | |
| Unidentified RT = 28:04-30.69 | - | - | 0.05 a | - | - | - | |
| Phenolic Compounds | |||||||
| Phenols | Vanillin | - | - | 0.07 a | - | 0.01 a | - |
| Rhododendrol | 0.01 a | - | - | - | - | - | |
| 3,4-dihydroxybenzaldehyde | 0.01 a | - | - | - | - | - | |
| 3-methoxy-4-OH-cinnamaldehyde | 0.06 a | - | 0.05 a | - | - | - | |
| Gallic acid | 0.32 ± 0.02 | 0.01 a | - | - | 0.01 a | - | |
| Protocatechuic acid | 0.13 a | - | - | - | - | - | |
| Tyrosol | 0.24 ± 0.01 | - | - | - | - | - | |
| Dihydroconiferyl alcohol | 0.28 ± 0.01 | - | 0.04 a | - | - | - | |
| Syringic acid | 0.05 a | - | - | 0.01 a | - | - | |
| Vanillic acid | 0.07 a | - | 0.01 a | 0.01 a | - | - | |
| 4-hydroxybenzoic acid | 0.13 a | - | - | - | - | 0.01 a | |
| Phenolic acid | Sinapyl alcohol | 0.24 ± 0.02 | - | 0.04 a | 0.01 a | - | - |
| 1-guaiacyl-3-OH-1-propanone | 0.03 a | - | - | - | - | - | |
| 1-guaiacyl-2-OH-1-ethanone | 0.03 a | - | 0.01 a | - | - | - | |
| 1-syringylglycerol | 0.04 a | - | 0.01 a | 0.03 a | 0.01 a | 0.01 a | |
| 1-guaiacyl -2,3-diOH-1-propanone | 0.04 a | - | 0.01 a | - | 0.01 a | 0.01 a | |
| 1-guaiacylglycerol | 0.16 ± 0.01 | - | - | - | - | - | |
| 1,3-(bis-guaiacyl)-1,2-propandiol | 0.09 a | - | - | - | - | 0.01 a | |
| P-hydroxyphenyl glycerol | 0.04 a | - | - | - | - | 0.01 a | |
| Coniferyl alcohol | 0.25 ± 0.03 | - | 0.03 a | 0.01 a | - | - | |
| Stilbenes and diarylheptanoids | 4,4′-diOH-3,3′-dimethoxystilbene | 0.01 a | - | - | 0.02 a | 0.01 a | - |
| X-c4h4(oh)3; c5h6(oh)3 | 0.23 ± 0.02 | - | 0.02 a | 0.01a | 0.01 a | - | |
| Lignans | Resorcinol | 0.10 ± 0.02 | - | - | - | - | - |
| Olivil | 0.01 a | - | 0.01 a | - | - | - | |
| glucopyranoside2[-4(OH)phe] | 0.02 a | - | - | - | - | - | |
| HMR | - | - | - | 0.01 a | - | - | |
| Todolactol | 0.17 ± 0.03 | - | 0.01 a | 0.01 a | 0.01 a | 0.01 a | |
| Flavonoids | |||||||
| tannins | Ellagic acid | 0.04 a | - | 0.01 a- | 0.01 a | - | - |
| Flavonoids | Pinobanksin | 0.01 a | - | - | - | - | - |
| Pinocembrin | 0.06 a | - | - | 0.02 a | 0.01 a | - | |
| (+)-Catechin | 0.02 a | - | 0.02 a | - | - | 0.01 a | |
| Dihydromyricetin | 0.03 a | - | - | - | - | - | |
| Quercetin | 0.05 a | - | 0.03 a | - | - | - | |
| Taxifolin | 0.21 ± 0.04 | - | - | 0.01 a | - | - | |
| Gallocatechin | 0.22 ± 0.02 | - | - | 0.06 a | 0.02 a | - | |
| 3′,4′,5′,3,X-PentaOH-dyhydroflavanol | 0.07 a | - | - | - | 0.01 a | 0.01 a | |
| Monomethyl gallocatechin | 0.02 a | - | - | - | - | - | |
| Other Compounds | |||||||
| Sugar derivatives | Monosaccharides | 6.84 ± 1.02 | - | 0.88 ± 0.08 | 0.01 a | 0.01 a | - |
| Sugar acids | 1.20 ± 0.09 | - | 0.06 a | - | - | - | |
| Sugar alcohol | 1.17 ± 0.06 | - | 0.01 a | - | - | - | |
| Others | Carboxyl acid | 1.12 ± 0.14 | - | - | - | - | - |
| Alpha-lapachone | 0.01 a | - | 0.02 a | - | - | - | |
| Dehydro-alpha-lapachone | 0.07 a | - | 0.10 ± 0.01 | - | - | - | |
| Hydroquinone- β-d-glucopyranoside | 0.06 a | - | - | - | - | - | |
| 3-OH-2-methyl-pyran-4-one | 0.01 a | - | 0.01 a | 0.02 a | - | - | |
| Non-identified | 0.13 ± 0.03 | - | 0.24 ± 0.03 | - | - | - | |
| Sample | Fraction | Extraction Yield (%) | Total Phenolic Content (mg GAE/g) | Antioxidant Activity IC50 Values (µg/mL) | ARP (1/IC50) |
|---|---|---|---|---|---|
| Hazelnuts shells | Raw Extract | - | 41.75 ± 0.62 a | 7.27 ± 0.19 a | 0.14 |
| 97:3 (1) | 82.32 ± 4.58 a | 31.37 ± 0.48 b | 6.34 ± 0.25 a | 0.16 | |
| 97:3 (2) | 5.39 ± 1.35 a | 33.62 ± 0.20 b | 4.62 ± 0.35 a | 0.22 | |
| 97:3 (3) | 4.21 ± 0.80 b | 48.46 ± 0.98 a | 2.85 ± 0.58 b | 0.35 | |
| 96:4 | 4.21 ± 0.65 b | 59.03 ± 1.23 a | 2.60 ± 0.32 b | 0.38 | |
| 95:5 | 3.88 ± 0.46 a | 83.04 ± 3.05 a | 1.10 ± 0.07 a | 0.91 | |
| Walnuts shells | Raw Extract | - | 13.14 ± 0.18 a | 7.82 ± 0.62 a | 0.13 |
| 97:3 (1) | 0.82 ± 0.07 a | nd | nd | - | |
| 97:3 (2) | 85.41 ± 4.65 a | 5.1 ± 0.01 a | very low | - | |
| 97:3 (3) | 7.57 ± 0.84 a | 63.60 ± 1.36 a | 7.17 ± 0.44 a | 0.14 | |
| 96:4 | 3.51 ± 0.13 b | 88.82 ± 4.06 a | 3.14 ± 0.22 a | 0.32 | |
| 95:5 | 2.70 ± 0.10 b | 49.10 ± 0.89 a | 5.81 ± 0.26 a | 0.17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, R.; Hemming, J.; Smeds, A.; Gordobil, O.; Willför, S.; Labidi, J. Recovery of Bioactive Compounds from Hazelnuts and Walnuts Shells: Quantitative–Qualitative Analysis and Chromatographic Purification. Biomolecules 2020, 10, 1363. https://doi.org/10.3390/biom10101363
Herrera R, Hemming J, Smeds A, Gordobil O, Willför S, Labidi J. Recovery of Bioactive Compounds from Hazelnuts and Walnuts Shells: Quantitative–Qualitative Analysis and Chromatographic Purification. Biomolecules. 2020; 10(10):1363. https://doi.org/10.3390/biom10101363
Chicago/Turabian StyleHerrera, René, Jarl Hemming, Annika Smeds, Oihana Gordobil, Stefan Willför, and Jalel Labidi. 2020. "Recovery of Bioactive Compounds from Hazelnuts and Walnuts Shells: Quantitative–Qualitative Analysis and Chromatographic Purification" Biomolecules 10, no. 10: 1363. https://doi.org/10.3390/biom10101363