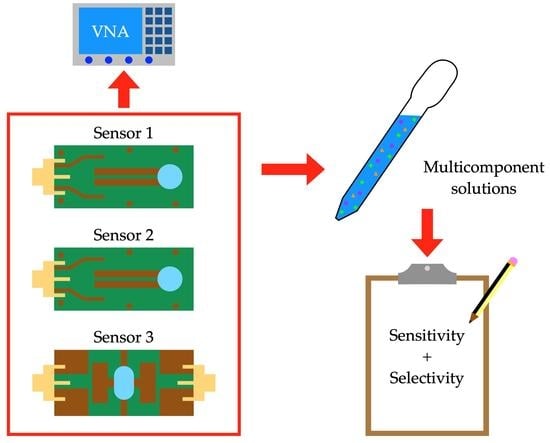
On the Selectivity of Planar Microwave Glucose Sensors with Multicomponent Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sensors under Study
2.2. Experimental Procedure
2.2.1. Biological Solutions
2.2.2. Measurements and Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shokrekhodaei, M.; Quinones, S. Review of non-invasive glucose sensing techniques: Optical, electrical and breath acetone. Sensors 2020, 20, 1251. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Thalmayer, A.S.; Zeising, Z.; Fischer, G.; Lübke, M. Commercial and scientific solutions for blood glucose monitoring—A review. Sensors 2022, 22, 425. [Google Scholar] [CrossRef] [PubMed]
- Guadarrama-Fernández, L.; Novell, M.; Blondeau, P.; Andrade, F.J. A disposable, simple, fast and low-cost paper-based biosensor and its application to the determination of glucose in commercial orange juices. Food Chem. 2018, 265, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Shabnam, L.; Faisal, S.N.; Roy, A.K.; Haque, E.; Minett, A.I.; Gomes, V.G. Doped graphene/Cu nanocomposite: A high sensitivity non-enzymatic glucose sensor for food. Food Chem. 2017, 221, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Cappon, G.; Acciaroli, G.; Vettoretti, M.; Facchinetti, A.; Sparacino, G. Wearable continuous glucose monitoring sensors: A revolution in diabetes treatment. Electronics 2017, 6, 65. [Google Scholar] [CrossRef] [Green Version]
- Tarasov, S.E.; Plekhanova, Y.V.; Rai, M.; Reshetilov, A.N. Nano- and microelectrochemical biosensors for determining blood glucose. In Macro, Micro, and Nano-Biosensors; Rai, M., Reshetilov, A., Plekhanova, Y., Ingle, A.P., Eds.; Springer: Cham, Switzerland, 2021; pp. 265–284. [Google Scholar] [CrossRef]
- Rghioui, A.; Lloret, J.; Harane, M.; Oumnad, A. A smart glucose monitoring system for diabetic patient. Electronics 2020, 9, 678. [Google Scholar] [CrossRef]
- Galant, A.L.; Kaufman, R.C.; Wilson, J.D. Glucose: Detection and analysis. Food Chem. 2015, 188, 149–160. [Google Scholar] [CrossRef]
- Hwang, D.-W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef]
- Dong, Q.; Ryu, H.; Lei, Y. Metal oxide based non-enzymatic electrochemical sensors for glucose detection. Electrochim. Acta 2021, 370, 137744. [Google Scholar] [CrossRef]
- Shen, N.; Xu, H.; Zhao, W.; Zhao, Y.; Zhang, X. Highly responsive and ultrasensitive non-enzymatic electrochemical glucose sensor based on Au foam. Sensors 2019, 19, 1203. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Wright, C.; Dincel, O.; Chi, T.-Y.; Kameoka, J. A low-cost paper glucose sensor with molecularly imprinted polyaniline electrode. Sensors 2020, 20, 1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oviedo, S.; Vehí, J.; Calm, R.; Armengol, J. A review of personalized blood glucose prediction strategies for T1DM patients. Int. J. Numer. Meth. Biomed. 2017, 33, e2833. [Google Scholar] [CrossRef]
- Georga, E.I.; Príncipe, J.C.; Fotiadis, D.I. Short-term prediction of glucose in type 1 diabetes using kernel adaptive filters. Med. Biol. Eng. Comput. 2019, 57, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Zale, A.; Mathioudakis, N. Machine Learning models for inpatient glucose prediction. Curr. Diabetes Rep. 2022, 22, 353–364. [Google Scholar] [CrossRef]
- Tankasala, D.; Linnes, J.C. Noninvasive glucose detection in exhaled breath condensate. Transl. Res. 2019, 213, 1–22. [Google Scholar] [CrossRef]
- Boubin, M.; Shrestha, S. Microcontroller Implementation of support vector machine for detecting blood glucose levels using breath volatile organic compounds. Sensors 2019, 19, 2283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Zhang, W.; Wang, M.L. An on-chip disposable salivary glucose sensor for diabetes control. J. Diabetes Sci. Technol. 2016, 10, 1344–1352. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhu, X.; Ju, Y.; Ma, B.; Zhao, C.; Liu, H. Electrocatalytic oxidation of glucose on bronze for monitoring of saliva glucose using a smart toothbrush. Sens. Actuator B Chem. 2019, 285, 56–61. [Google Scholar] [CrossRef]
- Badugu, R.; Reece, E.A.; Lakowicz, J.R. Glucose-sensitive silicone hydrogel contact lens toward tear glucose monitoring. J. Biomed. Opt. 2018, 23, 057005. [Google Scholar] [CrossRef] [PubMed]
- Geelhoed-Duijvestijn, P.; Vegelyte, D.; Kownacka, A.; Anton, N.; Joosse, M.; Wilson, C. Performance of the prototype NovioSense noninvasive biosensor for tear glucose in type 1 diabetes. J. Diabetes Sci. Technol. 2021, 15, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Jernelv, I.L.; Strøm, K.; Hjelme, D.R.; Aksnes, A. Mid-infrared spectroscopy with a fiber-coupled tuneable quantum cascade laser for glucose sensing. In Proceedings Volume 11233, Optical Fibers and Sensors for Medical Diagnostics and Treatment Applications XX; SPIE BiOS: San Francisco, CA, USA, 2020. [Google Scholar] [CrossRef]
- Delbeck, S.; Heise, M.H. Evaluation of opportunities and limitations of mid-infrared skin spectroscopy for noninvasive blood glucose monitoring. J. Diabetes Sci. Technol. 2021, 15, 19–27. [Google Scholar] [CrossRef]
- Aloraynan, A.; Rassel, S.; Xu, C.; Ban, D. A single wavelength mid-infrared photoacoustic spectroscopy for noninvasive glucose detection using machine learning. Biosensors 2022, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Huang, J.; Zhu, J.; Liang, S. An accurate noninvasive blood glucose measurement system using portable near-infrared spectrometer and transfer learning framework. IEEE Sens. J. 2021, 21, 3506–3519. [Google Scholar] [CrossRef]
- Hina, A.; Saadeh, W. Noninvasive blood glucose monitoring systems using near-infrared technology—A review. Sensors 2022, 22, 4855. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, P.; Chatterjee, B.; Sen, S. EM-wave biosensors: A review of RF, microwave, mm-wave and optical sensing. Sensors 2019, 19, 1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, T.; Foster, R.; Hao, Y. Radio-frequency and microwave techniques for non-invasive measurement of blood glucose levels. Diagnostics 2019, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Juan, C.G.; Potelon, B.; Quendo, C.; Bronchalo, E. Microwave planar resonant solutions for glucose concentration sensing: A systematic review. Appl. Sci. 2021, 11, 7018. [Google Scholar] [CrossRef]
- Buonanno, G.; Brancaccio, A.; Costanzo, S.; Solimene, R. Spectral methods for response enhancement of microwave resonant sensors in continuous non-invasive blood glucose monitoring. Bioengineering 2022, 9, 156. [Google Scholar] [CrossRef]
- Juan, C.G.; Bronchalo, E.; Torregrosa, G.; Ávila, E.; García, N.; Sabater-Navarro, J.M. Dielectric characterization of water glucose solutions using a transmission/reflection line method. Biomed. Signal Process. Control 2017, 31, 139–147. [Google Scholar] [CrossRef]
- Lin, T.; Gu, T.; Lasri, T. Highly sensitive characterization of glucose aqueous solution with low concentration: Application to broadband dielectric spectroscopy. Sens. Actuator A Phys. 2017, 267, 318–326. [Google Scholar] [CrossRef]
- Turgul, V.; Kale, I. Permittivity extraction of glucose solutions through artificial neural networks and non-invasive microwave glucose sensing. Sens. Actuator A Phys. 2018, 277, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Weatherbee, A.; Popov, I.; Vitkin, A. Accurate viscosity measurements of flowing aqueous glucose solutions with suspended scatterers using a dynamic light scattering approach with optical coherence tomography. J. Biomed. Opt. 2017, 22, 087003. [Google Scholar] [CrossRef] [PubMed]
- Grant, E.H. Relationship between relaxation time and viscosity for water. J. Chem. Phys. 1957, 26, 1575–1577. [Google Scholar] [CrossRef]
- Levy, E.; Puzenko, A.; Kaatze, U.; Ishai, P.B.; Feldman, Y. Dielectric spectra broadening as the signature of dipole-matrix interaction. I. Water in nonionic solutions. J. Chem. Phys. 2012, 136, 114502. [Google Scholar] [CrossRef]
- Mayani, M.G.; Herraiz-Martínez, F.J.; Domingo, J.M.; Giannetti, R. Resonator-based microwave metamaterial sensors for instrumentation: Survey, classification, and performance comparison. IEEE Trans. Instrum. Meas. 2021, 70, 9503414. [Google Scholar] [CrossRef]
- Camli, B.; Yalcinkaya, A.D. Resonant type RF glucose biosensors. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Shubair, R.M.; Ngo, K.; Alquié, G.; Deshours, F.; Kokabi, H. Multiple-cell microfluidic dielectric resonator for liquid sensing applications. IEEE Sens. J. 2021, 21, 6094–6104. [Google Scholar] [CrossRef]
- Govind, G.; Akhtar, M.J. Design of an ELC resonator-based reusable RF microfluidic sensor for blood glucose estimation. Sci. Rep. 2020, 10, 18842. [Google Scholar] [CrossRef]
- Verma, A.; Bhushan, S.; Tripathi, P.N.; Goswami, M.; Singh, B.R. A defected ground split ring resonator for an ultra- fast, selective sensing of glucose content in blood plasma. J. Electromagn. Waves Appl. 2017, 31, 1049–1061. [Google Scholar] [CrossRef]
- Vélez, P.; Mata-Contreras, J.; Dubuc, D.; Grenier, K.; Martín, F. Solute concentration measurements in diluted solutions by means of split ring resonators. In Proceedings of the 48th European Microwave Conference (EuMC), Madrid, Spain, 23–27 September 2018; pp. 231–234. [Google Scholar] [CrossRef] [Green Version]
- Zidane, M.A.; Rouane, A.; Hamouda, C.; Amar, H. Hyper-sensitive microwave sensor based on split ring resonator (SRR) for glucose measurement in water. Sens. Actuator A Phys. 2021, 321, 112601. [Google Scholar] [CrossRef]
- Chretiennot, T.; Dubuc, D.; Grenier, K. Microwave-based microfluidic sensor for non-destructive and quantitative glucose monitoring in aqueous solution. Sensors 2016, 16, 1733. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, A.; Coromina, J.; Muñoz-Enano, J.; Vélez, P.; Scott, J.; Ghorbani, K.; Martín, F. Highly sensitive phase-variation dielectric constant sensor based on a capacitively-loaded slow-wave transmission line. IEEE Trans. Circuits Syst. I-Regul. Pap. 2021, 68, 2787–2799. [Google Scholar] [CrossRef]
- Abdolrazzaghi, M.; Nayyeri, V.; Martín, F. Techniques to improve the performance of planar microwave sensors: A review and recent developments. Sensors 2022, 22, 6946. [Google Scholar] [CrossRef]
- Huang, S.Y.; Omkar; Yoshida, Y.; Garcia, A.; Chia, X.; Mu, W.C.; Meng, Y.S.; Yu, W. Microstrip line-based glucose sensor for noninvasive continuous monitoring using the main field for sensing and multivariable crosschecking. IEEE Sens. J. 2019, 19, 535–547. [Google Scholar] [CrossRef]
- Zeising, S.; Kirchner, J.; Khalili, H.F.; Ahmed, D.; Lübke, M.; Thalmayer, A.; Fischer, G. Towards realisation of a non-invasive blood glucose sensor using microstripline. In Proceedings of the 2020 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Dubrovnik, Croatia, 25–28 May 2020. [Google Scholar] [CrossRef]
- Juan, C.G.; Potelon, B.; Quendo, C.; García-Martínez, H.; Ávila-Navarro, E.; Bronchalo, E.; Sabater-Navarro, J.M. Study of Qu-based resonant microwave sensors and design of 3-D-printed devices dedicated to glucose monitoring. IEEE Trans. Instrum. Meas. 2021, 70, 8005716. [Google Scholar] [CrossRef]
- Harnsoongnoen, S.; Wanthong, A.; Charoen-In, U.; Siritaratiwat, A. Planar microwave sensor for detection and discrimination of aqueous organic and inorganic solutions. Sens. Actuator B Chem. 2018, 271, 300–305. [Google Scholar] [CrossRef]
- Juan, C.G.; Bronchalo, E.; Potelon, B.; Quendo, C.; Ávila-Navarro, E.; Sabater-Navarro, J.M. Concentration Measurement of microliter-volume water–glucose solutions using Q factor of microwave sensors. IEEE Trans. Instrum. Meas. 2019, 68, 2621–2634. [Google Scholar] [CrossRef]
- He, X.; Hao, X.; Yan, S.; Wu, F.; Jiang, J. Biosensing using an asymmetric split-ring resonator at microwave frequency. Integr. Ferroelectr. 2016, 172, 142–146. [Google Scholar] [CrossRef]
- Choi, H.; Naylon, J.; Luzio, S.; Beutler, J.; Birchall, J.; Martin, C.; Porch, A. Design and in vitro interference test of microwave noninvasive blood glucose monitoring sensor. IEEE Trans. Microw. Theory Tech. 2015, 63, 3016–3025. [Google Scholar] [CrossRef] [Green Version]
- Kiani, S.; Rezaei, P.; Fakhr, M. Dual-frequency microwave resonant sensor to detect noninvasive glucose-level changes through the fingertip. IEEE Trans. Instrum. Meas. 2021, 70, 6004608. [Google Scholar] [CrossRef]
- García, H.; Juan, C.G.; Ávila-Navarro, E.; Bronchalo, E.; Sabater-Navarro, J.M. Portable device based on microwave resonator for noninvasive blood glucose monitoring. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 1115–1118. [Google Scholar] [CrossRef]
- Juan, C.G.; García, H.; Ávila-Navarro, E.; Bronchalo, E.; Galiano, V.; Moreno, Ó.; Orozco, D.; Sabater-Navarro, J.M. Feasibility study of portable microwave microstrip open-loop resonator for noninvasive blood glucose level sensing: Proof of concept. Med. Biol. Eng. Comput. 2019, 57, 2389–2405. [Google Scholar] [CrossRef]
- Alahnomi, R.A.; Zakaria, Z.; Yussof, Z.M.; Althuwayb, A.A.; Alhegazi, A.; Alsariera, H.; Rahman, N.A. Review of recent microwave planar resonator-based sensors: Techniques of complex permittivity extraction, applications, open challenges and future research directions. Sensors 2021, 21, 2267. [Google Scholar] [CrossRef]
- Nguyen, M.A.; Bien, F.; Byun, G. Design of an out-folded patch antenna with a zeroth-order resonance for non-invasive continuous glucose monitoring. IEEE Trans. Antennas Propag. 2022, 70, 8932–8940. [Google Scholar] [CrossRef]
- Juan, C.G.; Bronchalo, E.; Potelon, B.; Álvarez-Pastor, J.; Sabater-Navarro, J.M. Use of coplanar quarter-wave resonators for glucose sensing in aqueous solutions. In Proceedings of the 2020 IEEE MTT-S International Microwave Biomedical Conference (IMBioC), Toulouse, France, 14–17 December 2020. [Google Scholar] [CrossRef]
- Turgul, V.; Kale, I. RF/microwave non-invasive blood glucose monitoring: An overview of the limitations, challenges & state-of-the-art. In Proceedings of the 2019 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 21–23 November 2019. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, W.-S.; Wang, D.-W. Miniaturized microwave microfluidic sensor based on spoof localized surface plasmons. In Proceedings of the 2022 IEEE MTT-S International Microwave Biomedical Conference (IMBioC), Suzhou, China, 16–18 May 2022. [Google Scholar] [CrossRef]
- Turgul, V.; Kale, I. Sensitivity of non-invasive RF/microwave glucose sensors and fundamental factors and challenges affecting measurement accuracy. In Proceedings of the 2018 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Houston, TX, USA, 14–17 May 2018. [Google Scholar] [CrossRef]
- Juan, C.G.; Potelon, B.; Quendo, C.; Bronchalo, E.; Sabater-Navarro, J.M. Highly-sensitive glucose concentration sensor exploiting inter-resonators couplings. In Proceedings of the 49th European Microwave Conference (EuMC), Paris, France, 1–3 October 2019; pp. 662–665. [Google Scholar] [CrossRef]
- Buonanno, G.; Brancaccio, A.; Costanzo, S.; Solimene, R. A forward-backward iterative procedure for improving the resolution of resonant microwave sensors. Electronics 2021, 10, 2930. [Google Scholar] [CrossRef]
- Casacuberta, P.; Vélez, P.; Muñoz-Enano, J.; Su, L.; Gil, M.; Ebrahimi, A.; Martín, F. Circuit analysis of a coplanar waveguide (CPW) terminated with a step-impedance resonator (SIR) for highly sensitive one-port permittivity sensing. IEEE Access 2022, 10, 62597–62612. [Google Scholar] [CrossRef]
- Peveler, W.J.; Yazdani, M.; Rotello, V.M. Selectivity and specificity: Pros and cons in sensing. ACS Sens. 2016, 1, 1282–1285. [Google Scholar] [CrossRef] [Green Version]
- Juan, C.G.; Bronchalo, E.; Potelon, B.; Quendo, C.; Sabater-Navarro, J.M. Glucose concentration measurement in human blood plasma solutions with microwave sensors. Sensors 2019, 19, 3779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, M.; Tajima, T.; Seyama, M. Broadband dielectric spectroscopy for quantitative analysis of glucose and albumin in multicomponent aqueous solution. IEEE J. Electromagn. RF Microw. Med. Biol. 2022, 6, 86–93. [Google Scholar] [CrossRef]
- Nguenouho, O.S.B.; Chevalier, A.; Potelon, B.; Benedicto, J.; Quendo, C. Dielectric characterization and modelling of aqueous solutions involving sodium chloride and sucrose and application to the design of a bi-parameter RF-sensor. Sci. Rep. 2022, 12, 7209. [Google Scholar] [CrossRef]
- Govind, G.; Akhtar, M.J. Metamaterial-inspired microwave microfluidic sensor for glucose monitoring in aqueous solutions. IEEE Sens. J. 2019, 19, 11900–11907. [Google Scholar] [CrossRef]
- Hong, J.-S.; Lancaster, M.J. Theory and experiment of novel microstrip slow-wave open-loop resonator filters. IEEE Trans. Microw. Theory Tech. 1997, 45, 2358–2365. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Sepulveda, L.V.; Olvera-Cervantes, J.L.; Saavedra, C.E. Multifrequency coupled-resonator sensor for dielectric characterization of liquids. IEEE Trans. Instrum. Meas. 2021, 70, 8005507. [Google Scholar] [CrossRef]
- Cao, Y.; Ruan, C.; Chen, K.; Zhang, X. Research on a high-sensitivity asymmetric metamaterial structure and its application as microwave sensor. Sci. Rep. 2022, 12, 1255. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.W.Y.; Kandwal, A.; Cheng, Q.; Shi, H.; Tobore, I.; Nie, Z. Non-invasive blood glucose monitoring using a curved Goubau line. Electronics 2019, 8, 662. [Google Scholar] [CrossRef] [Green Version]
- Carr, J.H. Clinical Hematology Atlas, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780323711920. [Google Scholar]
- Cao, R.; Komura, F.; Nonaka, A.; Kato, T.; Fukumashi, J.; Matsui, T. Quantitative Analysis of d-(+)-glucose in fruit juices using diffusion ordered-1H nuclear magnetic resonance spectroscopy. Anal. Sci. 2014, 30, 383–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrickx, H.; Verburggen, R.; Rosseneu-Motreff, M.Y.; Blaton, V.; Peeters, H. The dipolar origin of protein relaxation. Biochem. J. 1968, 110, 419–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanase, K.; Arai, R.; Sato, T. Intermolecular interactions and molecular dynamics in bovine serum albumin solutions studied by small angle X-ray scattering and dielectric relaxation spectroscopy. J. Mol. Liq. 2014, 200, 59–66. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and absorption in dielectrics I. Alternating current characteristics. J. Chem. Phys. 1941, 9, 341–451. [Google Scholar] [CrossRef] [Green Version]
- Kwok, R.S.; Liang, J.-F. Characterization of high-Q resonators for microwave-filter applications. IEEE Trans. Microw. Theory Tech. 1999, 47, 111–114. [Google Scholar] [CrossRef]
- Bray, J.R.; Roy, L. Measuring the unloaded, loaded, and external quality factors of one- and two-port resonators using scattering-parameter magnitudes at fractional power levels. IEE Proc.-Microw. Antennas Propag. 2004, 151, 345–350. [Google Scholar] [CrossRef]
- Hong, J.-S. Coupled resonator circuits. In Microstrip Filters for RF/Microwave Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 235–272. ISBN 9780470408773. [Google Scholar]
- Ebrahimi, A.; Scott, J.; Ghorbani, K. Ultrahigh-sensitivity microwave sensor for microfluidic complex permittivity measurement. IEEE Trans. Microw. Theory Tech. 2019, 67, 4269–4277. [Google Scholar] [CrossRef]
- Yunos, M.F.A.M.; Manczak, R.; Guines, C.; Mansor, A.F.M.; Mak, W.C.; Khan, S.; Ramli, N.A.; Pothier, A.; Nordin, A.N. RF remote blood glucose sensor and a microfluidic vascular phantom for sensor validation. Biosensors 2021, 11, 494. [Google Scholar] [CrossRef]
- Saeed, K.; Guyette, A.C.; Hunter, I.C.; Pollard, R.D. Microstrip resonator technique for measuring dielectric permittivity of liquid solvents and for solution sensing. In Proceedings of the 2007 IEEE/MTT-S International Microwave Symposium (IMS), Honolulu, HI, USA, 3–8 June 2007; pp. 1185–1188. [Google Scholar] [CrossRef]
- Grant, E.H.; Keefe, S.E.; Takashima, S. The dielectric behavior of aqueous solutions of bovine serum albumin from radiowave to microwave frequencies. J. Phys. Chem. 1968, 72, 4373–4380. [Google Scholar] [CrossRef]
- Bone, S.; Gascoyne, P.R.C.; Pethig, R. Dielectric properties of hydrated proteins at 9.9 GHz. J. Chem. Soc.-Faraday Trans. 1977, 73, 1605–1611. [Google Scholar] [CrossRef]
- Muñoz-Enano, J.; Peytral-Rieu, O.; Vélez, P.; Dubuc, D.; Grenier, K.; Martín, F. Characterization of the denaturation of bovine serum albumin (BSA) protein by means of a differential-mode microwave microfluidic sensor based on slot resonators. IEEE Sens. J. 2022, 22, 14075–14083. [Google Scholar] [CrossRef]
- Kurtinaitienė, B.; Razumienė, J.; Gurevičienė, V.; Melvydas, V.; Marcinkevičienė, L.; Bachmatova, I.; Meškys, R.; Laurinavičius, V. Application of oxygen-independent biosensor for testing yeast fermentation capacity. Biosens. Bioelectron. 2010, 26, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.K.; Jha, A.K.; Singh, S.P.; Akhter, Z.; Varshney, P.K.; Akhtar, M.J. Generalized multimode SIW cavity-based sensor for retrieval of complex permittivity of materials. IEEE Trans. Microw. Theory Tech. 2018, 66, 3063–3072. [Google Scholar] [CrossRef]
- Aquino, A.; Juan, C.G.; Potelon, B.; Quendo, C. Dielectric permittivity sensor based on planar open-loop resonator. IEEE Sens. Lett. 2021, 5, 3500204. [Google Scholar] [CrossRef]









| Parameter | Value | Parameter | Value |
|---|---|---|---|
| α | 150 | f | 0.40 |
| a | 3.40 | g | 0.80 |
| b | 3.00 | h | 9.70 |
| c | 1.60 | Rin (CS1) | 0.55 |
| d | 0.40 | Rin (CS2) | 0.30 |
| e | 1.60 | Rout | 1.20 |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| a | 2.10 | f | 1.70 |
| b | 2.00 | g | 5.10 |
| c | 0.60 | h | 4.00 |
| d | 2.00 | i | 4.60 |
| e | 1.00 | j | 10.70 |
| Set of Solutions | Fixed NaCl Concentration | Fixed Albumin Concentration | Different Glucose Concentrations |
|---|---|---|---|
| A0 | 0.6 | 0.0 | 0.0, 1.5, 3.0, 4.5, 6.0 |
| A2 | 0.6 | 2.0 | 0.0, 1.5, 3.0, 4.5, 6.0 |
| A3 | 0.6 | 3.0 | 0.0, 1.5, 3.0, 4.5, 6.0 |
| A4 | 0.6 | 4.0 | 0.0, 1.5, 3.0, 4.5, 6.0 |
| A5 | 0.6 | 5.0 | 0.0, 1.5, 3.0, 4.5, 6.0 |
| Parameter | Water–Glucose Solution [33] | Water–Albumin Solution [78] 1 |
|---|---|---|
| ε∞ | 4.3 | 6.0 |
| εs | 79.7 | 74.6 |
| τ (ps) | 11.1 | 8.3 |
| α | neglected | 0.07 |
| Sensor | fr0 (GHz) | Qu0 | k0 |
|---|---|---|---|
| CS1 | 4.359 | 47.219 | — |
| CS2 | 4.334 | 83.191 | — |
| 2RS | 4.228 | 43.866 | 217.77 × 10−4 |
| Set of Solutions | CS1 SQu (/g/dL) | CS1 RS(G)Qu (%/%) | CS2 SQu (/g/dL) | CS2 RS(G)Qu (%/%) | 2RS SQu (/g/dL) | 2RS RS(G)Qu (%/%) | 2RS Sk (/g/dL) | 2RS RS(G)k (%/%) |
|---|---|---|---|---|---|---|---|---|
| water | 0.384 | 0.799 | 0.743 | 0.869 | 1.962 | 4.115 | 2.49 × 10−4 | 1.228 |
| A0 | 0.338 | 0.716 | 0.657 | 0.790 | 1.957 | 4.108 | 2.17 × 10−4 | 0.996 |
| A2 | 0.327 | 0.693 | 0.642 | 0.772 | 1.747 | 3.963 | 2.09 × 10−4 | 0.960 |
| A3 | 0.319 | 0.676 | 0.630 | 0.757 | 1.351 | 3.011 | 2.16 × 10−4 | 0.992 |
| A4 | 0.313 | 0.663 | 0.627 | 0.754 | 1.283 | 2.860 | 2.14 × 10−4 | 0.983 |
| A5 | 0.306 | 0.648 | 0.619 | 0.744 | 1.195 | 2.663 | 2.08 × 10−4 | 0.955 |
| Set of Solutions | CS1 LOD (g/dL) | CS2 LOD (g/dL) | 2RS LOD (g/dL) |
|---|---|---|---|
| A0 | 0.6222 | 0.2434 | 0.0575 |
| A2 | 0.6541 | 0.2435 | 0.0668 |
| A3 | 0.6564 | 0.2738 | 0.0826 |
| A4 | 0.6939 | 0.2531 | 0.0851 |
| A5 | 0.7509 | 0.2651 | 0.0982 |
| Sensor | Type of Solutions | Sample Volume | fr0 (GHz) | Qu0 | RS(G)Qu (%/%) | LOD (g/dL) | Remarks |
|---|---|---|---|---|---|---|---|
| [85] | Binary | 5 µL | 1.11 | 187.14 | 0.186 | No data | — |
| [50] | Binary | 200 µL | 2.45 | 60.00 | 0.948 | No data | — |
| [52] | Binary | No data | 4.50 | 116.18 | 1.511 | No data | — |
| [49] | Binary | 46 µL | 4.62 | 22.00 | 2.727 | 0.103 | Biocompatible |
| [51] | Binary | 25 µL | 5.16 | 60.65 | 0.978 | 0.356 | — |
| [67] | Blood plasma multicomponent | 25 µL | 5.17 | 58.16 | From 0.829 to 0.346 | No data | Variations of glucose, ascorbic acid and lactic acid |
| CS1 [59] | Binary | 5 µL | 4.35 | 47.51 | 0.799 | 0.546 | — |
| CS2 [59] | Binary | 5 µL | 4.36 | 84.97 | 0.869 | 0.214 | — |
| 2RS [63] | Binary | 5 µL | 4.23 | 47.63 | 4.115 | 0.056 | — |
| CS1 here | Aqueous multicomponent | 5 µL | 4.36 | 47.219 | From 0.716 to 0.648 | From 0.622 to 0.751 | Variations of glucose and albumin |
| CS2 here | Aqueous multicomponent | 5 µL | 4.33 | 83.191 | From 0.790 to 0.744 | From 0.243 to 0.265 | Variations of glucose and albumin |
| 2RS here | Aqueous multicomponent | 5 µL | 4.23 | 43.866 | From 4.108 to 2.663 | From 0.058 to 0.098 | Variations of glucose and albumin |
| Set of Solutions | CS1 Error (g/dL) | CS1 Error (mg/dL) | CS2 Error (g/dL) | CS2 Error (mg/dL) | 2RS Error (g/dL) | 2RS Error (mg/dL) |
|---|---|---|---|---|---|---|
| A0 | 0.0000 | 0.000 | 0.0000 | 0.000 | 0.0000 | 0.000 |
| A2 | 0.0325 | 32.544 | 0.0228 | 22.831 | 0.0562 | 56.231 |
| A3 | 0.0562 | 56.213 | 0.0411 | 41.096 | 0.3096 | 309.640 |
| A4 | 0.0740 | 73.964 | 0.0457 | 45.662 | 0.3444 | 344.450 |
| A5 | 0.0947 | 94.675 | 0.0578 | 57.839 | 0.3892 | 389.241 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juan, C.G.; Bronchalo, E.; Potelon, B.; Quendo, C.; Muñoz, V.F.; Ferrández-Vicente, J.M.; Sabater-Navarro, J.M. On the Selectivity of Planar Microwave Glucose Sensors with Multicomponent Solutions. Electronics 2023, 12, 191. https://doi.org/10.3390/electronics12010191
Juan CG, Bronchalo E, Potelon B, Quendo C, Muñoz VF, Ferrández-Vicente JM, Sabater-Navarro JM. On the Selectivity of Planar Microwave Glucose Sensors with Multicomponent Solutions. Electronics. 2023; 12(1):191. https://doi.org/10.3390/electronics12010191
Chicago/Turabian StyleJuan, Carlos G., Enrique Bronchalo, Benjamin Potelon, Cédric Quendo, Víctor F. Muñoz, José M. Ferrández-Vicente, and José M. Sabater-Navarro. 2023. "On the Selectivity of Planar Microwave Glucose Sensors with Multicomponent Solutions" Electronics 12, no. 1: 191. https://doi.org/10.3390/electronics12010191