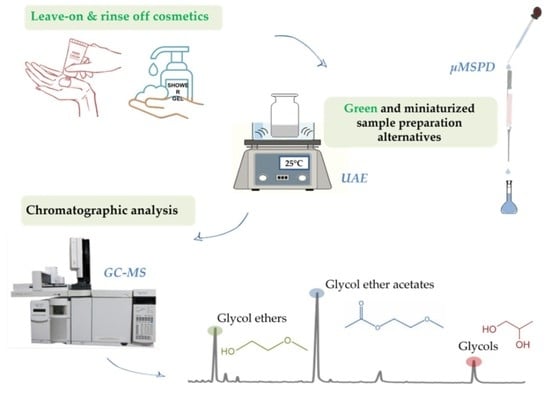
Miniaturized Sample Preparation Methods to Simultaneously Determine the Levels of Glycols, Glycol Ethers and Their Acetates in Cosmetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Materials
2.2. Cosmetic Samples
2.3. UAE Procedure
2.4. Micro-MSPD Procedure
2.5. GC-MS Analysis
2.6. Analytical Quality Parameters
3. Results and Discussion
3.1. Chromatographic Separation
3.2. UAE and µMSPD-GC-MS Performance
3.3. Comparison with Other Methodologies
3.4. Application to Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Ketttenis, P. The historic and current use of glycol ethers: A picture of change. Toxicol. Lett. 2005, 156, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Boatman, R.J. International industry initiatives to improve the glycol ether health effects knowledge base. Toxicol. Lett. 2005, 156, 39–50. [Google Scholar] [CrossRef] [PubMed]
- European Regulation (EC). No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetics products. Off. J. Eur. Union. 2009, 342, 59–209. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32009R1223 (accessed on 9 September 2021).
- Johanson, G. Toxicity review of ethylene glycol monomethyl ether and its acetate ester. Crit. Rev. Toxicol. 2000, 30, 307–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-S.; Ohtani, K.; Suda, M.; Kitagawa, K.; Nakayama, K.; Kawamoto, T.; Nakajima, T. Reproductive toxicity of ethylene glycol monoethyl ether in Aldh2 knockout mice. Ind. Health 2007, 45, 574–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venier, M.; Adami, G.; Larese, F.; Maina, G.; Renzi, N. Percutaneous absorption of 5 glycol ethers through human skin in vitro. Toxicol. In Vitro 2004, 18, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, X.; Xun, Z.; Du, W.; Guo, X.; Xian, Y.; Lin, S.; Tan, J. Simultaneous determination of glycol ethers and their acetates in cosmetics by gas chromatography with mass spectrometry. J. Sep. Sci. 2018, 41, 2354–2359. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Villa, C.; Neuhoff, C.; Dorato, S. Derivatization Procedure and HPLC Determination of 2-ethoxyethanol in Cosmetic Samples. Int. J. Cosmet. Sci. 1999, 21, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Celeiro, M.; Garcia-Jares, C.; Llompart, M.; Lores, M. Recent Advances in Sample Preparation for Cosmetics and Personal Care Products Analysis. Molecules 2021, 26, 4900. [Google Scholar] [CrossRef] [PubMed]
- Lores, M.; Llompart, M.; Alvarez-Rivera, G.; Guerra, E.; Vila, M.; Celeiro, M.; Lamas, J.P.; Garcia-Jares, C. Positive lists of cosmetic ingredients: Analytical methodology for regulatory and safety controls—A review. Anal. Chim. Acta 2016, 915, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Lores, M.; Celeiro, M.; Rubio, L.; Llompart, M.; Garcia-Jares, C. Extreme cosmetics and borderline products: An analytical-based survey of European regulation compliance. Anal. Bioanal. Chem. 2018, 410, 7085–7102. [Google Scholar] [CrossRef] [PubMed]
- Celeiro, M.; Vazquez, L.; Lamas, J.P.; Vila, M.; Garcia-Jares, C.; Llompart, M. Miniaturized Matrix Solid-Phase Dispersion for the Analysis of Ultraviolet Filters and Other Cosmetic Ingredients in Personal Care Products. Separations 2019, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Celeiro, M.; Rubio, L.; Garcia-Jares, C.; Lores, M. Multi-Target Strategy to Uncover Unexpected Compounds in Rinse-Off and Leave-On Cosmetics. Molecules 2021, 26, 2504. [Google Scholar] [CrossRef] [PubMed]
- Gembus, V.; Goullé, J.-P.; Lacroix, C. Determination of glycols in biological specimens by gas chromatography-mass spectrometry. J. Anal. Toxicol. 2002, 26, 280–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshikawa, M.; Tani, C. Sensitive determination of alkoxyethanols by pre-column derivatization with 1-anthroylnitrile and reversed-phasehigh-performance liquid chromatography. J. Chromatogr. A 2003, 1005, 215–221. [Google Scholar] [CrossRef]
- Giesen, Y.; Friedrich, C.; Breuer, D.; Fauss, J.; Hebisch, R.; Brock, T.H.; Hartwig, A.; Commission, M.A.K. Glycol esters, glycol ethers-Method for the determination of propylene glycol monoethyl ether, 1-ethoxy-2-propanol acetate, diethylene glycol monomethyl ether, diethylene glycol monoethyl ether and diethylene glycol monobutyl ether in workplace air using gas chromatography [Air Monitoring Methods, 2018]. In The MAK-Collection for Occupational Health and Safety: Annual Thresholds and Classifications for the Workplace; Wiley Online Library: Hoboken, NJ, USA, 2002; Volume 4, pp. 285–302. [Google Scholar]
- Kawakami, T.; Isama, K.; Tanaka-Kagawa, T.; Jinnno, H. Analysis of glycols, glycol ethers, and other volatile organic compounds present in household water-based hand pump sprays. J. Environ. Sci. Health Part A 2017, 52, 1204–1210. [Google Scholar] [CrossRef]
- Pastor-Belda, M.; Campillo, N.; Hernández-Córdoba, M.; Viñas, P. Gas chromatography with mass spectrometry for the quantification of ethylene glycol ethers in different household cleaning products. J. Sep. Sci. 2016, 39, 2292–2299. [Google Scholar] [CrossRef] [PubMed]



| Acronym | Common Name | CAS | EU Restrictions [3] |
|---|---|---|---|
| Glycols | |||
| ETG a | Ethylene glycol | 107-21-1 | Allowed as a humectant, solvent, and for viscosity control |
| DEG a | Diethylene glycol | 111-46-6 | Forbidden, except as traces in ingredients (0.1%) |
| TMG a | Tetramethylene glycol | 110-63-4 | Allowed as solvent |
| Glycol ethers | |||
| EGME a | Ethylene glycol monomethyl ether | 109-86-4 | Forbidden |
| EGDME a | Ethylene glycol dimethyl ether | 110-71-4 | Forbidden |
| EGEE a | Ethylene glycol monoethyl ether | 110-80-5 | Forbidden |
| EGBE b | Ethylene glycol monobutyl ether | 111-76-2 | Forbidden in aerosol dispensers (sprays); 4% (oxidative hair dyes); 2% (non-oxidative hair dyes) |
| DEGME a | Diethylene glycol monomethyl ether | 111-77-3 | Forbidden |
| DEGDME a | Diethylene glycol dimethyl ether | 111-96-6 | Forbidden |
| DEGEE b | Diethylene glycol monoethyl ether | 111-90-0 | Forbidden in eye and oral products; 7% (oxidative hair dyes); 5% (non-oxidative hair dyes); 10% (other leave-on products); 2.6% (other non-spray products); 2.6% (sprays: fine fragrance, hair sprays, antiperspirants, deodorants). In all cases, ETG ≤ 0.1%. |
| DEGBE b | Diethylene glycol monobutyl ether | 112-34-5 | Forbidden in aerosol dispensers (sprays); 9% (solvent in hair dye products) |
| PGME a | Propylene glycol monomethyl ether | 1589-47-5 | Forbidden |
| TEGDME a | Triethylene glycol dimethyl ether | 112-49-2 | Forbidden |
| Glycol ether acetates | |||
| EGMEA a | Ethylene glycol monomethyl ether acetate | 110-49-6 | Forbidden |
| EGEEA a | Ethylene glycol monoethyl ether acetate | 111-15-9 | Forbidden |
| PGMEA a | Propylene glycol monomethyl ether acetate | 108-65-6 | Allowed as solvent |
| iPGMEAb a | Isopropylene glycol monomethyl ether acetate | 70657-70-4 | Forbidden |
| Compounds | Retention Time (Min) | Quantification m/z Ion | Identification m/z Ions |
|---|---|---|---|
| Glycols | |||
| ETG | 17.06 | 31 | 33, 43, 62 |
| DEG | 21.97 | 75 | 45, 76 |
| TMG | 21.18 | 71 | 44, 57 |
| Glycol ethers | |||
| EGME | 9.91 | 45 | 58, 76 |
| EGDME | 7.16 | 60 | 45, 90 |
| EGEE | 10.59 | 59 | 45, 72 |
| EGBE | 13.57 | 57 | 87, 100 |
| DEGME | 16.57 | 59 | 58, 90 |
| DEGDME | 12.32 | 59 | 58, 89 |
| DEGEE | 17.04 | 59 | 72, 104 |
| DEGBE | 19.56 | 57 | 75, 87, 100 |
| PGME | 10.32 | 59 | 60, 75 |
| TEGDME | 18.52 | 103 | 59, 89, 133 |
| Glycol ether acetates | |||
| EGMEA | 10.99 | 58 | 43, 73 |
| EGEEA | 11.64 | 72 | 59, 87 |
| PGMEA | 10.49 | 43 | 72, 87 |
| iPGMEA | 10.96 | 59 | 43, 72 |
| Compounds | Linearity | Precision, RSD % | Recovery, % a | LODs (μg g−1) b | ||||
|---|---|---|---|---|---|---|---|---|
| Linear Range (μg L−1) | R2 | Intra-Day | Inter-Day | UAE | µMSPD | UAE | µMSPD | |
| Glycols | ||||||||
| ETG | 2–2000 | 0.9987 | 9.1 | 7.7 | 91 ± 6 | 92 ± 18 | 0.20 | 0.10 |
| DEG | 2–2000 | 0.9992 | 4.6 | 3.9 | 109 ± 14 | 100 ± 16 | 0.30 | 0.40 |
| TMG | 2–2000 | 0.9999 | 2.2 | 14 | 93 ± 10 | 96 ± 7 | 0.45 | 0.40 |
| Glycol ethers | ||||||||
| EGME | 2–2000 | 0.9941 | 6.5 | 4.9 | 99 ± 9 | 95 ± 9 | 0.19 | 0.10 |
| EGDME | 5–2000 | 0.9992 | 8.0 | 8.1 | 100 ± 8 | 96 ± 6 | 0.43 | 0.44 |
| EGEE | 2–2000 | 0.9947 | 0.8 | 0.7 | 103 ± 10 | 101 ± 18 | 0.25 | 0.21 |
| EGBE | 2–2000 | 0.9977 | 4.2 | 3.0 | 100 ± 20 | 98 ± 13 | 0.25 | 0.14 |
| DEGME | 2–2000 | 0.9991 | 4.2 | 6.4 | 94 ± 12 | 97 ± 5 | 0.75 | 0.38 |
| DEGDME | 2–2000 | 0.9952 | 4.0 | 3.3 | 102 ± 8 | 108 ± 16 | 0.07 | 0.03 |
| DEGEE | 5–2000 | 0.9984 | 3.6 | 3.1 | 108 ± 12 | 102 ± 4 | 0.75 | 0.43 |
| DEGBE | 5–2000 | 0.9988 | 0.1 | 5.6 | 99 ± 1 | 97 ± 15 | 0.50 | 0.30 |
| PGME | 2–2000 | 0.9959 | 8.0 | 5.8 | 104 ± 11 | 103 ± 18 | 0.25 | 0.11 |
| TEGDME | 2–2000 | 0.9991 | 13 | 9.4 | 100 ± 2 | 91 ± 4 | 0.30 | 0.31 |
| Glycol ether acetates | ||||||||
| EGMEA | 5–2000 | 0.9955 | 0.6 | 3.3 | 95 ± 4 | 99 ± 12 | 0.55 | 0.25 |
| EGEEA | 5–2000 | 0.9980 | 9.8 | 7.2 | 98 ± 8 | 94 ± 11 | 0.60 | 0.33 |
| PGMEA | 2–2000 | 0.9935 | 3.6 | 2.7 | 101 ± 10 | 100 ± 13 | 0.20 | 0.08 |
| iPGMEA | 2–2000 | 0.9947 | 2.5 | 2.6 | 98 ± 3 | 102 ± 13 | 0.15 | 0.08 |
| Analytes | Matrix | Extraction Technique | Extraction Time | Analysis | Recovery (%) | LODs (µg g−1) | Year | Ref. |
|---|---|---|---|---|---|---|---|---|
| 10 glycol ethers and their acetates | Cosmetics (0.5 g) | SLE | 19 min | GC-MS | 80–105 | 0.09–0.59 | 2018 | [7] |
| EGME | Cosmetics (0.1 g) | SLE + derivatization | >3 h | HPLC-UV a | 84–89 | 0.6–7.6 | 1999 | [8] |
| 12 glycols, glycol ethers, and their acetates | Household water-based sprays (0.5 mL) | SPE | - | GC-MS | 42–103 | 0.04–1.3 | 2017 | [17] |
| 6 glycol ethers | Household cleaning products, detergents (2 g) | QuEChERS b | 5 min | GC-MS | 89–115 | 0.01–1 | 2016 | [18] |
| 17 glycols, glycol ethers, and their acetates | Cosmetics (0.1 g) | µMSPD, UAE | 10 min | GC-MS | 79–116 | 0.03–0.75 | 2021 | This work |
| Analytes | Liquid Soap | Solid Soap | Body Milk |
|---|---|---|---|
| ETG | 9.7 ± 0.8 | 8.2 ± 1.3 | 14 ± 3 |
| DEG | 7.0 ± 2.7 | 16 ± 3 | 15 ± 1 |
| DEGEE | ND | ND | 9.4 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celeiro, M.; Rubio, L.; Garcia-Jares, C.; Lores, M. Miniaturized Sample Preparation Methods to Simultaneously Determine the Levels of Glycols, Glycol Ethers and Their Acetates in Cosmetics. Cosmetics 2021, 8, 102. https://doi.org/10.3390/cosmetics8040102
Celeiro M, Rubio L, Garcia-Jares C, Lores M. Miniaturized Sample Preparation Methods to Simultaneously Determine the Levels of Glycols, Glycol Ethers and Their Acetates in Cosmetics. Cosmetics. 2021; 8(4):102. https://doi.org/10.3390/cosmetics8040102
Chicago/Turabian StyleCeleiro, Maria, Laura Rubio, Carmen Garcia-Jares, and Marta Lores. 2021. "Miniaturized Sample Preparation Methods to Simultaneously Determine the Levels of Glycols, Glycol Ethers and Their Acetates in Cosmetics" Cosmetics 8, no. 4: 102. https://doi.org/10.3390/cosmetics8040102