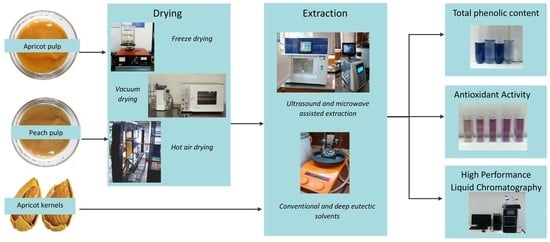
Sustainable Valorisation of Peach and Apricot Waste Using Green Extraction Technique with Conventional and Deep Eutectic Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Drying Pre-Treatment
2.2.1. Freeze Drying
2.2.2. Vacuum Drying
2.2.3. Hot Air Drying
2.2.4. Moisture Content Determination
2.2.5. Drying Kinetics Determination
2.3. Extraction
2.3.1. Preparation of DES and NADES
2.3.2. Ultrasound and Microwave Assisted Extraction (UMAE)
2.3.3. Calculation of the Extraction Yield (EY)
2.4. Characterisation of Extracts
2.4.1. Total Phenolic Content (TPC)
2.4.2. Total Flavonoid Content (TFC)
2.4.3. Antioxidant Activity (IC50)
2.4.4. Determination of Phenolic Composition by HPLC-DAD
2.5. Mathematical Modelling
2.6. Statistical Analysis
3. Results
3.1. Drying
3.2. Extraction
3.2.1. Extraction Yield (EY)
3.2.2. Antioxidant activity
3.2.3. Determination of Phenolic Composition by HPLC-DAD
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gupta, N.; Poddar, K.; Sarkar, D.; Kumari, N.; Padhan, B.; Sarkar, A. Fruit waste management by pigment production and utilization of residual as bioadsorbent. J. Environ. Manag. 2019, 244, 138–143. [Google Scholar] [CrossRef]
- Lau, K.Q.; Sabran, M.R.; Shafie, S.R. Utilization of Vegetable and Fruit By-products as Functional Ingredient and Food. Front. Nutr. 2021, 8, 661693. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.S.; Liew, R.K.; Lim, X.Y.; Ani, F.N.; Jusoh, A. Fruit waste as feedstock for recovery by pyrolysis technique. Int. Biodeterior. Biodegrad. 2016, 113, 325–333. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Scarano, P.; Sciarrillo, R.; Tartaglia, M.; Zuzolo, D.; Guarino, C. Circular economy and secondary raw materials from fruits as sustainable source for recovery and reuse. A review. Trends Food Sci. Technol. 2022, 122, 157–170. [Google Scholar] [CrossRef]
- Favre, G.; Hermosín-Gutiérrez, I.; Piccardo, D.; Gómez-Alonso, S.; González-Neves, G. Selectivity of pigments extraction from grapes and their partial retention in the pomace during red-winemaking. Food Chem. 2019, 277, 391–397. [Google Scholar] [CrossRef]
- Vázquez-González, M.; Fernández-Prior, Á.; Bermúdez Oria, A.; Rodríguez-Juan, E.M.; Pérez-Rubio, A.G.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Utilization of strawberry and raspberry waste for the extraction of bioactive compounds by deep eutectic solvents. LWT 2020, 130, 109645. [Google Scholar] [CrossRef]
- Koubaa, M.; Barba, F.J.; Grimi, N.; Mhemdi, H.; Koubaa, W.; Boussetta, N.; Vorobiev, E. Recovery of colorants from red prickly pear peels and pulps enhanced by pulsed electric field and ultrasound. Innov. Food Sci. Emerg. Technol. 2016, 37, 336–344. [Google Scholar] [CrossRef]
- Nishad, J.; Saha, S.; Kaur, C. Enzyme- and ultrasound-assisted extractions of polyphenols from Citrus sinensis (cv. Malta) peel: A comparative study. J. Food Process. Preserv. 2021, 43, e14046. [Google Scholar] [CrossRef]
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017, 34, 821–830. [Google Scholar] [CrossRef]
- Scarano, P.; Naviglio, D.; Prigioniero, A.; Tartaglia, M.; Postiglione, A.; Sciarrillo, R.; Guarino, C. Sustainability: Obtaining Natural Dyes from Waste Matrices Using the Prickly Pear Peels of Opuntia ficus-indica (L.) Miller. Agronomy 2020, 10, 528. [Google Scholar] [CrossRef] [Green Version]
- Scarano, P.; Tartaglia, M.; Zuzolo, D.; Prigioniero, A.; Guarino, C.; Sciarrillo, R. Recovery and Valorization of Bioactive and Functional Compounds from the Discarded of Opuntia ficus-indica (L.) Mill. Fruit Peel. Agronomy 2022, 12, 388. [Google Scholar] [CrossRef]
- Saidani, F.; Giménez, R.; Aubert, C.; Chalot, G.; Betrán, J.A.; Gogorcena, Y. Phenolic, sugar and acid profiles and the antioxidant composition in the peel and pulp of peach fruits. J. Food Compos. Anal. 2017, 62, 126–133. [Google Scholar] [CrossRef] [Green Version]
- FAOSTAT. 2020. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 21 April 2023).
- Iglesias-Carres, L.; Mas-Capdevila, A.; Bravo, F.I.; Bladé, C.; Arola-Arnal, A.; Muguerza, B. Optimization of extraction methods for characterization of phenolic compounds in apricot fruit (Prunus armeniaca). Food Funct. 2019, 10, 6492–6502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sochor, J.; Zitka, O.; Skutkova, H.; Pavlik, D.; Babula, P.; Krska, B.; Horna, A.; Adam, V.; Provaznik, I.; Kizek, R. Content of phenolic compounds and antioxidant capacity in fruits of apricot genotypes. Molecules 2010, 15, 6285–6305. [Google Scholar] [CrossRef] [Green Version]
- Mazza, K.E.L.; Santiago, M.C.P.A.; do Nascimento, L.S.M.; Godoy, R.L.O.; Souza, E.F.; Brígida, A.I.S.; Borguini, R.G.; Tonon, R.V. Syrah grape skin valorisation using ultrasound-assisted extraction: Phenolic compounds recovery, antioxidant capacity and phenolic profile. Int. J. Food Sci. Technol. 2019, 54, 641–650. [Google Scholar] [CrossRef]
- Galanakis, C.M. Emerging technologies for the production of nutraceuticals from agricultural by-products: A viewpoint of opportunities and challenges. Food Bioprod. Process. 2013, 91, 575–579. [Google Scholar] [CrossRef]
- Kumar, A.K.; Parikh, B.S.; Pravakar, M. Natural deep eutectic solvent mediated pretreatment of rice straw: Bioanalytical characterization of lignin extract and enzymatic hydrolysis of pretreated biomass residue. Environ. Sci. Pollut. Res. 2016, 23, 9265–9275. [Google Scholar] [CrossRef]
- Oomen, W.W.; Begines, P.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvent Extraction of Flavonoids of Scutellaria baicalensis as a Replacement for Conventional Organic Solvents. Molecules 2020, 25, 617. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Kong, Y.; Peng, J.; Song, X.; Che, X.; Liu, S.; Tian, W. Multivariate analysis of the process of deep eutectic solvent pretreatment of lignocellulosic biomass. Ind. Crops Prod. 2020, 150, 112363. [Google Scholar] [CrossRef]
- Mehariya, S.; Fratini, F.; Lavecchia, R.; Zuorro, A. Green extraction of value-added compounds form microalgae: A short review on natural deep eutectic solvents (NaDES) and related pre-treatments. J. Environ. Chem. Eng. 2021, 9, 105989. [Google Scholar] [CrossRef]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. Liquid structure of the choline chloride-urea deep eutectic solvent (reline) from neutron diffraction and atomistic modelling. Green Chem. 2016, 18, 2736–2744. [Google Scholar] [CrossRef] [Green Version]
- Francisco, M.; van den Bruinhorst, A.; Zubeir, L.F.; Peters, C.J.; Kroon, M.C. A new low transition temperature mixture (LTTM) formed by choline chloride+lactic acid: Characterization as solvent for CO2 capture. Fluid Phase Equilib. 2013, 340, 77–84. [Google Scholar] [CrossRef]
- Ali Redha, A. Review on Extraction of Phenolic Compounds from Natural Sources Using Green Deep Eutectic Solvents. J. Agric. Food Chem. 2021, 69, 878–912. [Google Scholar] [CrossRef]
- Naviglio, D.; Scarano, P.; Ciaravolo, M.; Gallo, M. Rapid Solid-Liquid Dynamic Extraction (RSLDE): A Powerful and Greener Alternative to the Latest Solid-Liquid Extraction Techniques. Foods 2019, 8, 245. [Google Scholar] [CrossRef] [Green Version]
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A.M. Microwave-assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chem. Eng. Process.-Process. Intensif. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Cassol, L.; Rodrigues, E.; Zapata Noreña, C.P. Extracting phenolic compounds from Hibiscus sabdariffa L. calyx using microwave assisted extraction. Ind. Crops Prod. 2019, 133, 168–177. [Google Scholar] [CrossRef]
- Stramarkou, M.; Papadaki, S.; Kyriakopoulou, K.; Krokida, M. Effect of drying and extraction conditions on the recovery of bioactive compounds from Chlorella vulgaris. J. Appl. Phycol. 2017, 29, 2947–2960. [Google Scholar] [CrossRef]
- Wang, J.; Sheng, K. Far-infrared and microwave drying of peach. LWT Food Sci. Technol. 2006, 39, 247–255. [Google Scholar] [CrossRef]
- Oliveira, E.G.; Rosa, G.S.; Moraes, M.A.; Pinto, L.A.A. Phycocyanin content of Spirulina Platensis dried in spouted bed and thin layer. J. Food Process. Eng. 2008, 31, 34–50. [Google Scholar] [CrossRef]
- Šumić, Z.; Vakula, A.; Tepić, A.; Čakarević, J.; Vitas, J.; Pavlić, B. Modeling and optimization of red currants vacuum drying process by response surface methodology (RSM). Food Chem. 2016, 203, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulou, K.; Pappa, A.; Krokida, M.; Detsi, A.; Kefalas, P. Effects of Drying and Extraction Methods on the Quality and Antioxidant Activity of Sea Buckthorn (Hippophae rhamnoides) Berries and Leaves. Dry. Technol. 2013, 31, 1063–1076. [Google Scholar] [CrossRef]
- Cunniff, P.A. Official Methods of Analysis of AOAC International. Assoc. Off. Anal. Chem. Int. 1998, 80, 127A. [Google Scholar]
- Krokida, M.K.; Karathanos, V.T.; Maroulis, Z.B.; Marinos-Kouris, D. Drying kinetics of some vegetables. J. Food Eng. 2003, 59, 391–403. [Google Scholar] [CrossRef]
- Vu, N.D.; Tran, N.T.Y.; Le, T.D.; Phan, N.T.M.; Doan, P.L.A.; Huynh, L.B.; Dao, P.T. Kinetic Model of Moisture Loss and Polyphenol Degradation during Heat Pump Drying of Soursop Fruit (Annona muricata L.). Processes 2022, 10, 2082. [Google Scholar] [CrossRef]
- Silva Donadone, D.B.; Giombelli, C.; Silva, D.L.G.; Stevanato, N.; Silva, C.; Bolanho Barros, B.C. Ultrasound-assisted extraction of phenolic compounds and soluble sugars from the stem portion of peach palm. J. Food Process. Preserv. 2020, 44, e14636. [Google Scholar] [CrossRef]
- Nipornram, S.; Tochampa, W.; Rattanatraiwong, P.; Singanusong, R. Optimization of low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) peel. Food Chem. 2018, 241, 338–345. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Vivas, N.; Glories, Y.; Lagune, L.; Cédric, S.; Augustin, M. Estimation du degré de polymérisation des procyanidines du raisin et du vin par la méthode au ρ-dimethylaminocinnamaldéhyde. J. Int. Sci. Vigne Vin 1994, 28, 319–336. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Stojanovic, B.T.; Mitic, S.S.; Stojanovic, G.S.; Mitic, M.N.; Kostic, D.A.; Paunovic, D.D.; Arsic, B.B. Phenolic profile and antioxidant activity of pulp and peel from peach and nectarine fruits. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Oikonomopoulou, V.; Stramarkou, M.; Plakida, A.; Krokida, M. Optimization of encapsulation of stevia glycosides through electrospraying and spray drying. Food Hydrocoll. 2022, 131, 107854. [Google Scholar] [CrossRef]
- Gigler, J.K.; Van Loon, W.K.P.; Vissers, M.M.; Bot, G.P.A. Forced convective drying of willow chips. Biomass Bioenergy 2000, 19, 259–270. [Google Scholar] [CrossRef]
- Şanal, I.S.; Güvenç, A.; Salgin, U.; Mehmetoǧlu, Ü.; Çalimli, A. Recycling of apricot pomace by supercritical CO2 extraction. J. Supercrit. Fluids 2004, 32, 221–230. [Google Scholar] [CrossRef]
- Ihns, R.; Diamante, L.M.; Savage, G.P.; Vanhanen, L. Effect of temperature on the drying characteristics, colour, antioxidant and beta-carotene contents of two apricot varieties. Int. J. Food Sci. Technol. 2011, 46, 275–283. [Google Scholar] [CrossRef]
- Knockaert, G.; Lemmens, L.; Van Buggenhout, S.; Hendrickx, M.; Van Loey, A. Changes in β-Carotene During Processing of Carrots. In Processing and Impact on Active Components in Food; Academic Press: Cambridge, MA, USA, 2014; ISBN 9780124047099. [Google Scholar]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Plazzotta, S.; Ibarz, R.; Manzocco, L.; Martín-Belloso, O. Optimizing the antioxidant biocompound recovery from peach waste extraction assisted by ultrasounds or microwaves. Ultrason. Sonochem. 2020, 63, 104954. [Google Scholar] [CrossRef]
- Setyaningsih, W.; Saputro, I.E.; Carrera, C.A.; Palma, M. Optimisation of an ultrasound-assisted extraction method for the simultaneous determination of phenolics in rice grains. Food Chem. 2019, 288, 221–227. [Google Scholar] [CrossRef]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Spigno, G.; Pizzorno, T.; De Faveri, D.M. Cellulose and hemicelluloses recovery from grape stalks. Bioresour. Technol. 2008, 99, 4329–4337. [Google Scholar] [CrossRef] [PubMed]
- Rozas, S.; Benito, C.; Alcalde, R.; Atilhan, M.; Aparicio, S. Insights on the water effect on deep eutectic solvents properties and structuring: The archetypical case of choline chloride + ethylene glycol. J. Mol. Liq. 2021, 344, 117717. [Google Scholar] [CrossRef]
- Kalab, M.; Lapsley, K. Microstructural Principles of Food Processing and Engineering; Trends in Food Science & Technology; Aguilera, J.M., Stanley, D.W., Eds.; Elsevier: Amsterdam, The Netherlands, 1990; p. xiv. 343p, ISBN-1 85166 421 1. [Google Scholar]
- Vorobyova, V.; Skiba, M.; Miliar, Y.; Frolenkova, S. Enhanced Phenolic Compounds Extraction From Apricot Pomace By Natural Deep Eutectic Solvent Combined With Ultrasonic-Assisted Extraction. J. Chem. Technol. Metall. 2021, 56, 919–931. [Google Scholar]
- Fan, X.; Jiao, W.; Wang, X.; Cao, J.; Jiang, W. Polyphenol composition and antioxidant capacity in pulp and peel of apricot fruits of various varieties and maturity stages at harvest. Int. J. Food Sci. Technol. 2018, 53, 327–336. [Google Scholar] [CrossRef]
- Göttingerová, M.; Kumšta, M.; Rampáčková, E.; Kiss, T.; Nečas, T. Analysis of phenolic compounds and some important analytical properties in selected apricot genotypes. HortScience 2021, 56, 1446–1452. [Google Scholar] [CrossRef]
- Andreotti, C.; Ravaglia, D.; Ragaini, A.; Costa, G. Phenolic compounds in peach (Prunus persica) cultivars at harvest and during fruit maturation. Ann. Appl. Biol. 2008, 153, 11–23. [Google Scholar] [CrossRef]
- Ding, T.; Cao, K.; Fang, W.; Zhu, G.; Chen, C.; Wang, X.; Wang, L. Evaluation of phenolic components (anthocyanins, flavanols, phenolic acids, and flavonols) and their antioxidant properties of peach fruits. Sci. Hortic. 2020, 268, 109365. [Google Scholar] [CrossRef]
- Kan, T.; Gundogdu, M.; Ercisli, S.; Muradoglu, F.; Celik, F.; Gecer, M.K.; Kodad, O.; Zia-Ul-Haq, M. Phenolic compounds and vitamins in wild and cultivated apricot (Prunus armeniaca L.) fruits grown in irrigated and dry farming conditions. Biol. Res. 2014, 47, 46. [Google Scholar] [CrossRef] [Green Version]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]





| Parameter | Coding Values/Real Values | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Extraction time (min) | 5 | 10 | 20 |
| Ultrasound Power (W) | 150 | 450 | 750 |
| Solvent/dry solid ratio (mL/g) | 10 | 20 | 30 |
| Biomass Type | Moisture Content Reduction -Wet Basis- (g H2O/100 g Solid) | Drying Constant, k (min−1) | TPC (mg GAE/g Dry Biomass) | TFC (mg CE/g Dry Biomass) | IC50 (/mg Dry Biomass) |
|---|---|---|---|---|---|
| Wet | - | - | 23.45 a ± 5.1 × 10−1 | 19.60 a ± 1.4 × 10−1 | 1.60 a ± 3.1 × 10−2 |
| Freeze dried | 80.71 a ± 7.3 × 10−1 | 1.33 × 10−2 b ± 2.3.0 × 10−3 | 30.08 b ± 5.2 × 10−1 | 9.63 b ± 1.3 × 10−1 | 0.98 b ± 3.1 × 10−2 |
| Vacuum dried | 80.55 a ± 5.9 × 10−1 | 1.86 × 10−2 b ± 6.0 × 10−3 | 25.42 c ± 3.4 × 10−1 | 9.29 b,c ± 1.3 × 10−1 | 1.54 c ± 1.3 × 10−1 |
| Hot air dried | 81.29 a ±1.1 × 10−1 | 3.71 × 10−2 a ± 8.3.0 × 10−3 | 21.30 d ± 3.9 × 10−1 | 9.18 c ± 1.2 × 10−1 | 1.99 d ± 7.9 × 10−2 |
| Biomass Type | Moisture Content Reduction -Wet Basis- (g H2O/100 g Solid) | Drying Constant, k (min−1) | TPC (mg GAE/g Dry Biomass) | TFC (mg CE/g Dry Biomass) | IC50 (/mg Dry Biomass) |
|---|---|---|---|---|---|
| Wet | - | - | 11.40 a ± 3.1 × 10−1 | 5.02 a ± 1.4 × 10−1 | 1.55 a ± 5.0 × 10−2 |
| Freeze dried | 89.54 a ± 8.1 × 10−1 | 1.61 × 10−2 b ± 4.0 × 10−3 | 19.31 b ± 2.1 × 10−1 | 8.12 b ± 1.3 × 10−1 | 1.33 a ± 5.1 × 10−2 |
| Vacuum dried | 89.02 a ± 4.3 × 10−1 | 1.93 × 10−2 b ± 4.0 × 10−3 | 16.64 c ± 2.3 × 10−1 | 5.59 b ± 1.3 × 10−1 | 2.55 b ± 2.7 × 10−2 |
| Hot air dried | 90.25 a ± 1.9 × 10−1 | 2.81 × 10−2 a ± 6.1 × 10−3 | 13.19 d ± 3.1 × 10−1 | 4.04 d ± 1.2 × 10−1 | 3.56 c ± 5.0 × 10−2 |
| a | at | aP | aR | R2 (%) | |
|---|---|---|---|---|---|
| Water | 12.34 ± 1.98 × 10−1 | 5.99 × 10−2 ± 4.04 × 10−2 | 2.96 × 10−1 ± 4.00 × 10−2 | −8.01 × 10−1 ± 3.75 × 10−2 | 95.03 |
| p-value | 0.00 | 0.25 | 0.00 | 0.00 | |
| Ethanol: water | 16.14 ± 4.59 × 10−1 | 2.22 × 10−1 ± 7.11 × 10−2 | 2.79 × 10−1 ± 7.03 × 10−2 | −6.88 × 10−1 ± 6.91 × 10−2 | 88.97 |
| p-value | 0.00 | 0.00 | 0.00 | 0.00 | |
| DES | 10.58 ± 2.51 × 10−1 | 1.23 × 10−1 ± 6.11 × 10−2 | 3.10 × 10−1 ± 6.11 × 10−2 | −4.25 × 10−1 ± 6.48 × 10−2 | 93.96 |
| p-value | 0.00 | 0.25 | 0.00 | 0.00 | |
| NaDES | 8.60 ± 1.51 × 10−1 | 3.68 × 10−1 ± 4.38 × 10−2 | 1.38 × 10−1 ± 4.05 × 10−2 | −2.62 × 10−1 ± 5.05 × 10−2 | 95.63 |
| p-value | 0.00 | 0.00 | 0.00 | 0.00 |
| a | at | aP | aR | R2 (%) | |
|---|---|---|---|---|---|
| Water | 10.51 ± 1.28 × 10−1 | 2.63 × 10−1 ± 3.14 × 10−2 | 2.59 × 10−1 ± 3.08 × 10−2 | −6.49 × 10−2 ± 3.83 × 10−2 | 94.12 |
| p-value | 0.00 | 0.00 | 0.00 | 0.12 | |
| Ethanol: water | 12.47 ± 3.80 × 10−1 | 2.69 × 10−1 ± 7.80 × 10−2 | 2.77 × 10−1 ± 7.74 × 10−2 | −2.08 × 10−1 ± 9.06 × 10−2 | 91.83 |
| p-value | 0.00 | 0.00 | 0.00 | 0.00 | |
| DES | 23.21 ± 1.16 × 10−1 | 2.24 × 10−1 ± 1.31 × 10−2 | 2.29 × 10−1 ± 1.26 × 10−2 | 1.73 × 10−1 ± 1.72 × 10−2 | 98.67 |
| p-value | 0.00 | 0.00 | 0.00 | 0.00 | |
| NaDES | 19.48 ± 3.26 × 10−1 | 2.76 × 10−1 ± 4.33 × 10−2 | 1.55 × 10−1 ± 4.00 × 10−2 | 2.66 × 10−1 ± 5.87 × 10−2 | 88.96 |
| p-value | 0.00 | 0.00 | 0.00 | 0.00 |
| a | at | aP | aR | R2 (%) | |
|---|---|---|---|---|---|
| Water | 8.13 ± 3.12 × 10−2 | 2.56 × 10−1 ± 9.89 × 10−2 | 1.20 × 10−1 ± 8.92 × 10−2 | −5.92 × 10−1 ± 1.21 × 10−2 | 96.89 |
| p-value | 0.00 | 0.00 | 0.00 | 0.00 | |
| Ethanol: water | 15.05 ± 2.56 × 10−1 | 2.14 × 10−1 ± 4.38 × 10−2 | 1.14 × 10−1 ± 3.92 × 10−2 | −2.63 × 10−1 ± 4.96 × 10−2 | 92.65 |
| p-value | 0.00 | 0.05 | 0.00 | 0.00 | |
| DES | 15.35 ± 1.99 × 10−1 | 1.17 × 10−1 ± 3.41 × 10−2 | 2.34 × 10-± 3.26 × 10−2 | −4.56 × 10−1 ± 4.14 × 10−2 | 93.84 |
| p-value | 0.00 | 0.05 | 0.00 | 0.30 | |
| NaDES | 13.90 ± 1.02 × 10−1 | 3.12 × 10−1 ± 1.87 × 10−2 | 1.11 × 10−1 ± 1.68 × 10−2 | −2.03 × 10−1 ± 2.18 × 10−2 | 97.62 |
| p-value | 0.00 | 0.00 | 0.00 | 0.00 |
| Extraction Conditions | Water | Ethanol: Water | DES | NaDES | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Solvent/Dry Solid Ratio (mL/g) | Time (min) | Ultrasound Power (W) | TPC (mg GAE/g Init. Dry Sol.) | IC50 (/mg Init. Dry Sol.) | TPC (mg GAE/g Init. Dry Sol.) | IC50 (/mg Init. Dry sol.) | TPC (mg GAE/g Init. Dry Sol.) | IC50 (/mg Init. Dry Sol.) | TPC (mg GAE/g Init. Dry Sol.) | IC50 (/mg Init. Dry Sol.) |
| 10 | 10 | 450 | 21.37 a ± 0.01 | 0.52 a ± 0.06 | 25.65 a ± 3.02 | 0.11a ± 0.05 | 13.89 a ± 1.51 | 2.02 a ± 0.25 | 10.26 a,d ± 1.21 | 2.25 a ± 0.05 |
| 20 | 5 | 450 | 12.29 b ± 0.51 | 2.10 b,d ± 0.42 | 13.52 b ± 0.56 | 0.78 b ± 0.06 | 9.83 b,d ± 0.41 | 3.12 b,c ± 0.21 | 6.76 b ± 0.28 | 5.38 b ± 0.45 |
| 20 | 10 | 150 | 8.56 c ± 0.63 | 3.44 c ± 0.38 | 11.98 b ± 0.88 | 1.35 c ± 0.31 | 7.70 c ± 0.56 | 3.70 b,d ± 0.25 | 7.47 b ± 0.12 | 4.18 c ± 0.21 |
| 20 | 10 | 450 | 12.90 b,d ± 0.89 | 1.56 d ± 0.16 | 18.00 c ± 0.58 | 0.37 a,b ± 0.09 | 11.00 b,d ± 1.67 | 1.70 a ± 0.09 | 8.36 b,c ± 0.11 | 4.16 c ± 0.11 |
| 20 | 10 | 750 | 13.87 d ± 0.78 | 2.28 b ± 0.05 | 18.76 c ± 2.03 | 0.77 b ± 0.08 | 12.62 a,d ± 0.51 | 2.64 c ± 0.12 | 9.38 a,c ± 1.02 | 4.02 c ± 0.31 |
| 20 | 20 | 450 | 13.28 b,d ± 0.21 | 1.58 d ± 0.16 | 18.59 c ± 0.29 | 0.39 a,b ± 0.08 | 11.62 a,b ± 0.29 | 2.80 c ± 0.12 | 11.16 d ± 0.17 | 1.92 a ± 0.06 |
| 30 | 10 | 450 | 8.37 c ± 0.11 | 3.70 c ± 0.21 | 10.88 b ± 0.14 | 1.25 c ± 0.25 | 8.08c ± 0.28 | 3.74 d ± 0.36 | 7.62 b ± 0.10 | 4.78 b,c ± 0.35 |
| TPC- IC50 correlation coefficients | −0.88 | −0.80 | −0.69 | −0.85 | ||||||
| Extraction Conditions | Water | Ethanol: Water | DES | NaDES | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Solvent/Dry Solid Ratio (mL/g) | Time (min) | Ultrasound Power (W) | TPC (mg GAE/g Init. Dry Sol.) | IC50 (/mg Init. Dry Sol.) | TPC (mg GAE/g Init. Dry Sol.) | IC50 (/mg Init. Dry Sol.) | TPC (mg GAE/g Init. Dry Sol.) | IC50 (/mg Init. Dry Sol.) | TPC (mg GAE/g Init. Dry Sol.) | IC50 (/mg Init. Dry Sol.) |
| 10 | 10 | 450 | 11.29 a ± 0.28 | 3.76 a ± 0.28 | 13.62 a ± 1.60 | 3.54 a ± 0.08 | 20.96 a ± 0.40 | 2.62 a ± 0.02 | 16.15 a ± 0.07 | 2.10 a ± 0.09 |
| 20 | 5 | 450 | 8.58 b ± 0.11 | 6.36 b ± 0.23 | 11.47 b,c ± 0.48 | 4.10 b ± 0.06 | 19.50 b ± 0.45 | 3.90 b ± 0.03 | 14.50 b ± 0.71 | 4.66 b ± 0.08 |
| 20 | 10 | 150 | 7.53 b ± 0.42 | 8.20 c ± 0.11 | 8.99 c ± 0.66 | 5.10 c ± 0.18 | 17.91 c ± 0.42 | 4.52 c ± 0.06 | 17.15 a ± 0.21 | 1.76 c ± 0.09 |
| 20 | 10 | 450 | 10.91 a ± 0.29 | 3.72 a ± 0.21 | 13.50 a,b ± 0.43 | 3.32 a ± 0.11 | 23.32 d ± 0.72 | 0.22 d ± 0.02 | 20.32 c ± 0.12 | 1.02 d,e ± 0.03 |
| 20 | 10 | 750 | 11.46 a,c ± 0.57 | 3.96 a ± 0.10 | 14.07 a ± 1.52 | 2.02 d ± 0.13 | 25.90 e,f ± 0.29 | 1.78 e ± 0.01 | 22.28 d ± 0.32 | 0.50 f ± 0.10 |
| 20 | 20 | 450 | 12.48 c ± 0.71 | 2.46 d ± 0.09 | 15.80 a ± 0.24 | 0.64 e ± 0.02 | 26.83 e ± 0.18 | 1.58 f ± 0.03 | 22.50 d ± 0.71 | 0.90 d ± 0.04 |
| 30 | 10 | 450 | 10.77 a ± 0.11 | 4.56 e ± 0.11 | 9.80 c ± 0.13 | 4.02 b ± 0.08 | 25.42 f ± 0.72 | 0.24 d ± 0.06 | 21.65 d ± 0.49 | 1.12 e ± 0.04 |
| TPC- IC50 correlation coefficients | −0.95 | −0.84 | −0.81 | −0.86 | ||||||
| Extraction Conditions | Water | Ethanol: Water | DES | NaDES | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Solvent/Dry Solid Ratio (mL/g) | Time (min) | Ultrasound Power (W) | TPC (mg GAE/g Init. Dry Sol.) | IC50 (/mg Init. Dry Sol.) | TPC (mg GAE/g Init. Dry Sol.) | IC50 (/mg Init. Dry Sol.) | TPC (mg GAE/g Init. Dry Sol.) | IC50 (/mg Init. Dry Sol.) | TPC (mg GAE/g Init. Dry Sol.) | IC50 (/mg Init. Dry Sol.) |
| 10 | 10 | 450 | 8.49 a ± 0.03 | 4.60 a ± 0.30 | 17.95 a ± 0.12 | 0.35 a ± 0.09 | 15.55 a ± 0.23 | 0.92 a,e ± 0.09 | 15.95 a ± 0.60 | 1.17 a ± 0.05 |
| 20 | 5 | 450 | 6.78 b ± 0.11 | 6.84 b ± 0.28 | 12.50 b ± 0.04 | 3.56 b ± 0.35 | 14.39 b ± 0.11 | 1.43 b ± 0.11 | 11.01 b ± 0.52 | 3.12 b ± 0.08 |
| 20 | 10 | 150 | 7.13 b ± 0.04 | 5.91 c ± 0.15 | 13.28 b ± 0.29 | 2.02 c ± 0.26 | 11.52 c ± 0.25 | 5.96 c ± 0.15 | 12.43 c ± 0.23 | 2.85 b,c ± 0.11 |
| 20 | 10 | 450 | 8.11 c ± 0.29 | 4.34 a ± 0.24 | 16.20 c ± 0.52 | 0.51 a,d ± 0.06 | 16.56 d ± 0.18 | 056 a ± 0.09 | 13.98 d ± 0.27 | 2.84 b,c ± 0.24 |
| 20 | 10 | 750 | 8.66 a ± 0.06 | 5.50 c ± 0.11 | 15.96 c ± 0.28 | 0.93 d,e ± 0.06 | 16.77 d ± 0.15 | 1.22 b,e ± 0.15 | 14.94 a,d ± 0.45 | 1.34 a ± 0.16 |
| 20 | 20 | 450 | 9.68 d ± 0.07 | 2.68 d ± 0.13 | 17.10 d ± 0.26 | 0.45 a,d ± 0.09 | 16.84 d ± 0.42 | 1.47 b ± 0.21 | 17.13 e ± 0.25 | 0.53 d ± 0.08 |
| 30 | 10 | 450 | 7.97 c ± 0.11 | 5.28 c ± 0.30 | 13.28 b ± 0.18 | 1.30 e ± 0.06 | 14.55 b ± 0.51 | 2.04 d ± 0.21 | 12.70 c ± 0.19 | 2.74 c ± 0.11 |
| TPC- IC50 correlation coefficients | −0.86 | −0.84 | −0.87 | −0.91 | ||||||
| Optimal Extraction Conditions | Chlorogenic Acid (mg/100 g) | Catechin (mg/100 g) | Epicatechin (mg/100 g) | Rutin (mg/100 g) | Quercetin (mg/100 g) | |
|---|---|---|---|---|---|---|
| Water | 10 (mL/g), 10 min, 450 W | 45.62 a ± 0.64 | 6.42 a ± 0.11 | 2.09 a ± 0.09 | 62.60 a ± 0.60 | 4.25 a ± 0.21 |
| Ethanol: water | 10 (mL/g), 10 min, 450 W | 46.65 a ± 0.82 | 10.36 b ± 0.09 | 4.11 b ± 0.11 | 71.66 b ± 0.71 | 6.05 b ± 0.33 |
| DES | 10 (mL/g), 10 min, 450 W | 20.09 b ± 0.06 | 6.01 a ± 0.41 | 4.01 b ± 0.08 | 24.10 c ± 0.10 | 1.07 c ± 0.06 |
| NaDES | 20 (mL/g), 20 min, 450 W | 18.00 c ± 0.30 | 6.41 a ± 0.40 | 6.60 c ± 0.60 | 22.71 c ± 0.51 | 0.82 c ± 0.09 |
| Optimal Extraction Conditions | Chlorogenic Acid (mg/100 g) | Catechin (mg/100 g) | Epicatechin (mg/100 g) | Rutin (mg/100 g) | Quercetin (mg/100 g) | |
|---|---|---|---|---|---|---|
| Water | 20 (mL/g), 20 min, 450 W | 14.51 a ± 0.55 | 1.38 a ± 0.21 | 1.01 a ± 0.21 | 15.62 a ± 0.14 | 1.23 a ± 0.15 |
| Ethanol: water | 20 (mL/g), 20 min, 450 W | 36.31 b ± 0.32 | 2.08 a ± 0.27 | 2.01 a,b ± 0.38 | 55.66 b ± 0.32 | 0.99 a ± 0.07 |
| DES | 20 (mL/g), 20 min, 450 W | 56.89 c ± 0.95 | 9.99 b ± 1.02 | 3.69 c ± 0.69 | 99.31 c ± 2.1 | 5.96 b ± 0.15 |
| NaDES | 20 (mL/g), 20 min, 450 W | 41.32 d ± 1.37 | 6.66 c ± 1.02 | 3.02 b,c ± 0.61 | 68.55 d ± 0.87 | 4.16 c ± 0.54 |
| Optimal Extraction Conditions | Chlorogenic Acid (mg/100 g) | Catechin (mg/100 g) | Epicatechin (mg/100 g) | Rutin (mg/100 g) | Quercetin (mg/100 g) | |
|---|---|---|---|---|---|---|
| Water | 20 (mL/g), 20 min, 450 W | 17.32 a ± 0.25 | 11.38 a ± 0.32 | 1.00 a ± 0.10 | 1.89 a ± 0.09 | 2.32 a ± 0.08 |
| Ethanol: water | 10 (mL/g), 10 min, 450 W | 60.00 b ± 1.8 | 46.92 b ± 1.47 | 4.01 b ± 0.60 | 1.46 a ± 0.08 | 7.03 b ± 0.15 |
| DES | 20 (mL/g), 20 min, 450 W | 61.00 b ± 2.95 | 54.71 c ± 2.33 | 4.40 b ± 1.29 | 9.32 b ± 2.1 | 5.81 b ± 1.7 |
| NaDES | 20 (mL/g), 20 min, 450 W | 56.32 b ± 2.08 | 41.81 d ± 1.33 | 4.02 b ± 1.10 | 2.50 a ± 0.65 | 3.18 a ± 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stramarkou, M.; Oikonomopoulou, V.; Panagiotopoulou, M.; Papadaki, S.; Krokida, M. Sustainable Valorisation of Peach and Apricot Waste Using Green Extraction Technique with Conventional and Deep Eutectic Solvents. Resources 2023, 12, 72. https://doi.org/10.3390/resources12060072
Stramarkou M, Oikonomopoulou V, Panagiotopoulou M, Papadaki S, Krokida M. Sustainable Valorisation of Peach and Apricot Waste Using Green Extraction Technique with Conventional and Deep Eutectic Solvents. Resources. 2023; 12(6):72. https://doi.org/10.3390/resources12060072
Chicago/Turabian StyleStramarkou, Marina, Vasiliki Oikonomopoulou, Margarita Panagiotopoulou, Sofia Papadaki, and Magdalini Krokida. 2023. "Sustainable Valorisation of Peach and Apricot Waste Using Green Extraction Technique with Conventional and Deep Eutectic Solvents" Resources 12, no. 6: 72. https://doi.org/10.3390/resources12060072