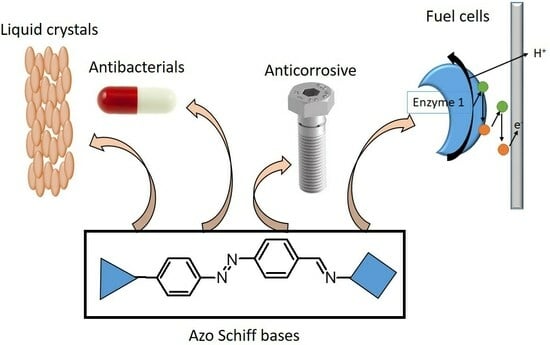
Azobenzene-Containing Schiff-Bases—Syntheses and Dyes Applications
Abstract
:1. Introduction
2. Syntheses and Structures of Azobenzene-Containing Schiff-Bases
2.1. Schiff Base Synthesis
2.1.1. Main Imine Syntheses
2.1.2. Aniline to Arylamines
2.1.3. Two Aldehydes and Aniline
2.1.4. Unusual Imine Synthesis
2.1.5. Complex Synthesis: Aldehyde Coordination First, Imine Synthesis on Metal
2.2. Main Azo Schiff Base Synthetic Method
2.2.1. Azobenzene Synthesis by Mills Reaction
2.2.2. Azo Schiff Base Synthesis by Diazotization Method
2.2.3. Structural Variations Due to Orientation When the Azo and Imine Groups Are in the Same Benzene Ring
2.2.4. Azoaldehyde, Schiff Base and Complex Synthesis
2.2.5. If There Are More than One Amino Group, Can a Schiff Base Ligand Be Formed First?
2.2.6. Adjustment of the Number of Imines
2.2.7. Unusual Reactions
3. Azobenzene-Containing Schiff-Bases Applications
3.1. Sensors
3.2. Liquid Crystals
3.3. Enzymatic Fuel Cells and Electrode Mediators
3.4. UV-Protection and Anti-Corrosive Effect
3.5. Anticancer and Antibacterials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamon, F.; Djedaini-Pilard, F.; Barbot, F.; Len, C. Azobenzenes—Synthesis and Carbohydrate Applications. Tetrahedron 2009, 65, 10105–10123. [Google Scholar] [CrossRef]
- Brown, E.V.; Kipp, W.H. Mechanism of the Base-Catalyzed Synthesis of Azobenzenes. J. Org. Chem. 1971, 36, 170–173. [Google Scholar] [CrossRef]
- Sinn, E.; Harris, C.M. Schiff Base Metal Complexes as Ligands1. Coord. Chem. Rev. 1969, 4, 391–422. [Google Scholar] [CrossRef]
- Merritt, I.C.; Jacquemin, D.; Vacher, M. Cis→ Trans Photoisomerisation of Azobenzene: A Fresh Theoretical Look. Phys. Chem. Chem. Phys. 2021, 23, 19155–19165. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.D.; Friss, T.R.; Enriquez, M.M.; Isley, W.; Incarvito, C.; Frank, H.A.; Gascon, J.; Burdette, S.C. Proof for the Concerted Inversion Mechanism in the Trans→cis Isomerization of Azobenzene Using Hydrogen Bonding To Induce Isomer Locking. J. Org. Chem. 2010, 75, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Crecca, C.R.; Roitberg, A.E. Theoretical Study of the Isomerization Mechanism of Azobenzene and Disubstituted Azobenzene Derivatives. J. Phys. Chem. A 2006, 110, 8188–8203. [Google Scholar] [CrossRef] [PubMed]
- Bafana, A.; Devi, S.S.; Chakrabarti, T. Azo Dyes: Past, Present and the Future. Environ. Rev. 2011, 19, 350–371. [Google Scholar] [CrossRef]
- Drillaud, N.; Banaszak-Léonard, E.; Pezron, I.; Len, C. Synthesis and Evaluation of a Photochromic Surfactant for Organic Reactions in Aqueous Media. J. Org. Chem. 2012, 77, 9553–9561. [Google Scholar] [CrossRef]
- Léonard, E.; Mangin, F.; Villette, C.; Billamboz, M.; Len, C. Azobenzenes and Catalysis. Catal. Sci. Technol. 2016, 6, 379–398. [Google Scholar] [CrossRef]
- Altomare, A.; Ciardelli, F.; Marchini, M.; Solaro, R. Polymeric Dispersions of Model Azobenzene Dyes. Polymer 2005, 46, 2086–2096. [Google Scholar] [CrossRef]
- Aubert, A.; Fayeulle, A.; Vayssade, M.; Billamboz, M.; Lénard, E. New Trends on Photoswitchable Antibiotics: From Syntheses to Applications. Photocatal. Res. Potential 2023, 1, 10007. [Google Scholar] [CrossRef]
- Pang, X.; Lv, J.; Zhu, C.; Qin, L.; Yu, Y. Photodeformable Azobenzene-containing Liquid Crystal Polymers and Soft Actuators. Adv. Mater. 2019, 31, 1904224. [Google Scholar] [CrossRef] [PubMed]
- Skačej, G.; Querciagrossa, L.; Zannoni, C. On the Effects of Different Trans and Cis Populations in Azobenzene Liquid Crystal Elastomers: A Monte Carlo Investigation. ACS Appl. Polym. Mater. 2023, 5, 5805–5815. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Zhao, H.; Chen, S. New Azobenzene Liquid Crystal with Dihydropyrazole Heterocycle and Photoisomerization Studies. R. Soc. Open Sci. 2020, 7, 200474. [Google Scholar] [CrossRef] [PubMed]
- Moss, G.P.; Smith, P.A.S.; Tavernier, D. Glossary of Class Names of Organic Compounds and Reactivity Intermediates Based on Structure (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67, 1307–1375. [Google Scholar] [CrossRef]
- Schiff, H. Mittheilungen Aus Dem Universitätslaboratorium in Pisa: Eine Neue Reihe Organischer Basen. Justus Liebigs Ann. Chem. 1864, 131, 118–119. [Google Scholar] [CrossRef]
- Larrow, J.F.; Jacobsen, E.N. Asymmetric Processes Catalyzed by Chiral (Salen) Metal Complexes. In Organometallics in Process Chemistry; Springer: Berlin/Heidelberg, Germany, 2004; pp. 123–152. [Google Scholar]
- Breinbauer, R.; Jacobsen, E.N. Cooperative Asymmetric Catalysis with Dendrimeric [Co (Salen)] Complexes. Angew. Chem. Int. Ed. 2000, 39, 3604–3607. [Google Scholar] [CrossRef]
- Wantulok, J.; Szala, M.; Quinto, A.; Nycz, J.E.; Giannarelli, S.; Sokolová, R.; Książek, M.; Kusz, J. Synthesis, Electrochemical and Spectroscopic Characterization of Selected Quinolinecarbaldehydes and Their Schiff Base Derivatives. Molecules 2020, 25, 2053. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, M.A.; Esakkiammal, M.; Mariappan, S.S.; Dharmaraja, J.; Jeyakumar, T. Synthesis, Characterization and Biocidal Activities of Some Schiff Base Metal Complexes. Indian J. Pharm. Sci. 2010, 72, 216–222. [Google Scholar] [CrossRef]
- Ramesh, R.; Maheswaran, S. Synthesis, spectra, dioxygen affinity and antifungal activity of Ru (III) Schiff base complexes. J. Inorg. Biochem. 2003, 96, 457–462. [Google Scholar] [CrossRef]
- Keskioğlu, E.; Gündüzalp, A.B.; Hamurcu, F.; Erk, B.C. Fe(III) and Co(III) Complexes of Tetradentate (ONNO) Schiff Base Ligands: Synthesis, Characterization, Properties and Biological Activity. Spectrochim. Acta Part A 2008, 70, 634–640. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Arif, M.; Sarfraz, M. Metal-based antibacterial and antifungal amino acid derived Schiff bases: Their synthesis, characterization and in vitro biological activity. Appl. Organometal. Chem. 2007, 21, 294–302. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Munawar, A.; Supuran, C.T. Transition Metal Ion Complexes of Schiff-Bases. Synthesis, Characterization and Antibacterial Properties. Met.-Based Drugs 2001, 8, 137–143. [Google Scholar] [CrossRef]
- Vamsikrishna, N.; Kumar, M.P.; Ramesh, G.; Ganji, N.; Daravath, S.; Shivaraj. DNA Interactions and Biocidal Activity of Metal Complexes of Benzothiazole Schiff Bases: Synthesis, Characterization and Validation. J. Chem. Sci. 2017, 5, 609–622. [Google Scholar] [CrossRef]
- Alnoman, R.; Al-Nazawi, F.K.; Ahmed, H.A.; Hagar, M.S. Optical, and Geometrical Approaches of New Natural Fatty Acids’ Esters/Schiff Base Liquid Crystals. Molecules 2019, 24, 4293. [Google Scholar] [CrossRef]
- Zolezzi, S.; Spodine, E.; Decinti, A.; Mohamed, G.G. Electrochemical Studies of Copper(II) Complexes with Schiff-Base Ligands. Polyhedron 2022, 21, 55–59. [Google Scholar] [CrossRef]
- Mohamed, G.G. Synthesis, Characterization and Biological Activity of Bis(Phenylimine) Schiff Base Ligands and Their Metal Complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 64, 188–195. [Google Scholar] [CrossRef]
- Souane, R.; Isel, F.; Peruch, F.; Lutz, P.J. Pyridine Bis(Imine) Cobalt or Iron Complexes for Ethylene and 1-Hexene (Co)Polymerisation. Comptes Rendus Chim. 2002, 5, 43–48. [Google Scholar] [CrossRef]
- Vicini, P.; Geronikaki, A.; Incerti, M.; Busonera, B.; Poni, G.; Cabras, C.A.; La Colla, P. Synthesis and Biological Evaluation of Benzo[d]Isothiazole, Benzothiazole and Thiazole Schiff Bases. Bioorg. Med. Chem. 2003, 11, 4785–4789. [Google Scholar] [CrossRef]
- Lee, J.; Melchakova, I.; Nayab, S.; Kim, K.; Ko, Y.H.; Yoon, M.; Avramov, P.; Lee, H. Synthesis and Characterization of Zinc (II), Cadmium (II), and Palladium (II) Complexes with the Thiophene-Derived Schiff Base Ligand. ACS Omega 2023, 8, 6016–6029. [Google Scholar] [CrossRef]
- Kizilkaya, H.; Dag, B.; Aral, T.; Genc, N.; Erenler, R. Synthesis, Characterization, and Antioxidant Activity of Heterocyclic Schiff Bases. J. Chin. Chem. Soc. 2020, 67, 1696–1701. [Google Scholar] [CrossRef]
- Issa, R.M.; Khedr, A.M.; Rizk, H.F. UV–vis, IR and 1H NMR spectroscopic studies of some Schiff bases derivatives of 4-aminoantipyrine. Spectrochim. Acta Part A 2005, 62, 621–629. [Google Scholar] [CrossRef]
- Asiri, A.M.; Khan, S. Synthesis and Anti-Bacterial Activities of Some Novel Schiff Bases Derived from Aminophenazone. Molecules 2010, 15, 6850–6858. [Google Scholar] [CrossRef]
- Xavier, A.; Srividhya, N. Synthesis and Study of Schiff Base Ligands. IOSR J. Appl. Chem. 2014, 7, 6–15. [Google Scholar] [CrossRef]
- Jarrahpour, A.; Khalili, D.; Clercq, E.D.; Salmi, C.; Brunel, J.M. Synthesis, Antibacterial Antifungal and Antiviral Activity Evaluation of Some New Bis-Schiff Bases of Isatin and Their Derivatives. Molecules 2007, 12, 1720–1730. [Google Scholar] [CrossRef]
- Paschke, R.; Liebsch, S.; Tschierske, C.; Oakley, M.A.; Sinn, E. Synthesis and Mesogenic Properties of Binuclear Copper(II) Complexes Derived from Salicylaldimine Schiff Bases. Inorg. Chem 2003, 42, 8230–8240. [Google Scholar] [CrossRef]
- Merino, E. Synthesis of Azobenzenes: The Coloured Pieces of Molecular Materials. Chem. Soc. Rev. 2011, 40, 3835–3853. [Google Scholar] [CrossRef]
- Fazekašová, S.; Gonda, J.; Martinková, M.; Pilátová, M.B.; Majirská, M.; Turčanová, V.; Jáger, D.T. Synthesis and anticancer profile of novel FTY720 analogues with azobenzene frameworks. Tetrahedron 2023, 137, 133391. [Google Scholar] [CrossRef]
- Aemissegger, A.; Hilvert, D. Synthesis and Application of an Azobenzene Amino Acid as a Light-Switchable Turn Element in Polypeptides. Nat. Protoc. 2007, 2, 161–167. [Google Scholar] [CrossRef]
- Nishioka, H.; Liang, X.; Asanuma, H. Effect of the Ortho Modification of Azobenzene on the Photoregulatory Efficiency of DNA Hybridization and the Thermal Stability of Its Cis Form. Chem.–A Eur. J. 2010, 16, 2054–2062. [Google Scholar] [CrossRef]
- Sørensen, J.; Hansen, E.J.; Larsen, D.; Elmquist, M.A.; Buchleithner, A.; Florean, L.; Beeren, S.R. Light-controlled enzymatic synthesis of γ-CD using a recyclable azobenzene template. Chem. Sci. 2023, 14, 7725–7732. [Google Scholar] [CrossRef]
- Priewisch, B.; Ruck-Braun, K. Efficient Preparation of Nitrosoarenes for the Synthesis of Azobenzenes. J. Org. Chem. 2005, 70, 2350–2352. [Google Scholar] [CrossRef]
- Yu, B.-C.; Shirai, Y.; Tour, J.M. Syntheses of New Functionalized Azobenzenes for Potential Molecular Electronic Devices. Tetrahedron 2006, 62, 10303–10310. [Google Scholar] [CrossRef]
- Hosgor, E.; Akdag, A. Synthesis of Azobenzene Containing Macrocycles Exhibiting Unexpected Fluorescence. Chem. Pap. 2022, 76, 3891–3898. [Google Scholar] [CrossRef]
- Fan, S.; Lam, Y.; He, L.; Xin, J.H. Synthesis and Photochromism of Catechol-Containing Symmetrical Azobenzene Compounds. R. Soc. Open Sci. 2022, 9, 211894. [Google Scholar] [CrossRef]
- Samanta, S.; Beharry, A.A.; Sadovski, O.; McCormick, T.M.; Babalhavaeji, A.; Tropepe, V.; Woolley, G.A. Photoswitching Azo Compounds In Vivo with Red Light. J. Am. Chem. Soc. 2013, 135, 9777–9784. [Google Scholar] [CrossRef]
- Antoine John, A.; Lin, Q. Synthesis of Azobenzenes Using N-Chlorosuccinimide and 1,8-Diazabicyclo[5.4.0]Undec-7-Ene (DBU). J. Org. Chem. 2017, 82, 9873–9876. [Google Scholar] [CrossRef]
- Blackburn, O.A.; Coe, B.J.; Fielden, J.; Helliwell, M.; McDouall, J.J.W.; Hutchings, M.G. Nickel(II) and Palladium(II) Complexes of Azobenzene-Containing Ligands as Dichroic Dyes. Inorg. Chem. 2010, 49, 9136–9150. [Google Scholar] [CrossRef]
- Kaur, H.; Lim, S.M.; Ramasamy, K.; Vasudevan, M.; Shah, S.A.A.; Narasimhan, B. Diazenyl Schiff Bases: Synthesis, Spectral Analysis, Antimicrobial Studies and Cytotoxic Activity on Human Colorectal Carcinoma Cell Line (HCT-116). Arab. J. Chem. 2020, 13, 377–392. [Google Scholar] [CrossRef]
- Slassi, S.; El-Ghayoury, A.; Aarjane, M.; Yamni, K.; Amine, A. New Copper(II) and Zinc(II) Complexes Based on Azo Schiffbase Ligand: Synthesis, Crystal Structure, Photoisomerization Study and Antibacterial Activity. Appl. Organomet. Chem. 2020, 34, 5503. [Google Scholar] [CrossRef]
- Gulcan, M.; Özdemir, S.; Dündar, A.; İspir, E.; Kurtoğlu, M. Mononuclear Complexes Based on Pyrimidine Ring Azo Schiff-Base Ligand: Synthesis, Characterization, Antioxidant, Antibacterial, and Thermal Investigations. Z. Anorg. Allg. Chem. 2014, 640, 1754–1762. [Google Scholar] [CrossRef]
- Jiao, T.; Li, X.; Li, Q.; Zhou, J. Optical Property and Photoisomerization of Some Functional Azobenzene Derivatives with Aromatic Substituted Groups. Appl. Mech. Mater. 2011, 121–126, 1009–1013. [Google Scholar] [CrossRef]
- Gao, C.; Ma, X.; Zhang, Q.; Wang, Q.; Qu, D.; Tian, H. A Light-Powered Stretch–Contraction Supramolecular System Based on Cobalt Coordinated [1]Rotaxane. Org. Biomol. Chem. 2011, 9, 1126–1132. [Google Scholar] [CrossRef]
- Alkam, H.; Alwan, A.W.; Al Shemary, R.K.R. Complexes of Co (II), Cu (II), Ni (II), Pt (II) And Pd (II) with N3O-Chelating Ligand Incorporating Azo and Schiff Base Moieties: Synthesis, Spectroscopic, Thermal Decomposition, Theoretical Studies, and Thermodynamic Parameters. Int. J. Pharm. Res. 2021, 13, 3370–3378. [Google Scholar]
- Slassi, S.; Aarjane, M.; Amine, A. Synthesis, molecular geometry, Hirshfeld surface analysis, spectroscopic (NMR, UV–visible), DFT and TD-DFT calculations of an azoimidazole-based Schiff base. J. Iran. Chem. Soc. 2022, 19, 4789–4801. [Google Scholar] [CrossRef]
- Kaler, S.; McKeown, P.; Ward, B.D.; Jones, M.D. Aluminium(III) and Zinc(II) Complexes of Azobenzene-Containing Ligands for Ring-Opening Polymerisation of ε-Caprolactone and Rac-Lactide. Inorg. Chem. Front. 2021, 8, 711–719. [Google Scholar] [CrossRef]
- Hassan, A.M.; Said, A.O.; Heakal, B.H.; Younis, A.; Aboulthana, W.M.; Mady, M.F. Green Synthesis, Characterization, Antimicrobial and Anticancer Screening of New Metal Complexes Incorporating Schiff Base. ACS Omega 2022, 7, 32418–32431. [Google Scholar] [CrossRef]
- Kumar, Y.; Singh, V.D.; Dwivedi, B.K.; Singh, N.K.; Pandey, D.S. Solid State Emissive Azo-Schiff Base Ligands and Their Zn(II) Complexes: Acidochromism and Photoswitching Behaviour. New J. Chem. 2021, 45, 199–207. [Google Scholar] [CrossRef]
- Nigam, N.; Kumar, S.; Dutta, P.K.; Pei, S.; Ghosh, T. Chitosan Containing Azo-Based Schiff Bases: Thermal, Antibacterial and Birefringence Properties for Bio-Optical Devices. RSC Adv. 2016, 6, 5575–5581. [Google Scholar] [CrossRef]
- Jarrahpour, A.A.; Motamedifar, M.; Pakshir, K.; Hadi, N.; Zarei, M. Synthesis of Novel Azo Schiff Bases and Their Antibacterial and Antifungal Activities. Molecules 2004, 9, 815–824. [Google Scholar] [CrossRef]
- Abid, K.K.; Al-barody, S.M. Synthesis, Characterisation and Liquid Crystalline Behaviour of Some Lanthanides Complexes Containing Two Azobenzene Schiff Base. Liq. Cryst. 2014, 41, 1303–1314. [Google Scholar] [CrossRef]
- Saeed, S.E.; Al-Harbi, T.M.; Alhakimi, A.N.; El-Hady, M.M.A. Synthesis and Characterization of Metal Complexes Based on Aniline Derivative Schiff Base for Antimicrobial Applications and UV Protection of a Modified Cotton Fabric. Coatings 2022, 12, 1181. [Google Scholar] [CrossRef]
- Tanaka, K.; Fukuoka, S.; Miyanishi, H.; Takahashi, H. Novel chiral Schiff base macrocycles containing azobenzene chromophore: Gelation and guest inclusion. Tetrahedron Lett. 2010, 51, 2693–2696. [Google Scholar] [CrossRef]
- Al-Adilee, K.J.; Hasan, S.R. Synthesis, Characterization and Biological Activity of Heterocyclic Azo-Schiff Base Ligand Derived from 2-Amino-5-Methyl Thiazol and Some Transition Metal Ions. IOP Conf. Ser. Earth Environ. Sci. 2021, 790, 012031. [Google Scholar] [CrossRef]
- Kumar, M.; Agarkar, H.; Degani, M.S. New Schiff Base-Linked Arylazopyrazoles as Reagents for the Photometric Detection of Fluoride Ions. J. Anal. Chem. 2023, 78, 866–877. [Google Scholar] [CrossRef]
- Ahmed, N.H.S.; Saad, G.R.; Ahmed, H.A.; Hagar, M. New Wide-Stability Four-Ring Azo/Ester/Schiff Base Liquid Crystals: Synthesis, Mesomorphic, Photophysical, and DFT Approaches. RSC Adv. 2020, 10, 9643–9656. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Wang, Q.; Zhao, Y.; Shang, L. Cholesteric Cellulose Liquid Crystal Ink for Three-Dimensional Structural Coloration. Proc. Natl. Acad. Sci. USA 2022, 119, e2204113119. [Google Scholar] [CrossRef]
- Hossain, M.A. Adaptive Camouflage Textiles with Thermochromic Colorant and Liquid Crystal for Multidimensional Combat Background, a Technical Approach for Advancement in Defence Protection. Am. J. Mater. Eng. Technol. 2021, 9, 31–47. [Google Scholar] [CrossRef]
- Yoon, C.; Choi, J.-H.; Kim, J.P. Improving the Contrast Ratio of Red Pixels in Liquid-Crystal Displays by Synthesizing Synergists from an Anthraquinone Colorant. Mol. Cryst. Liq. Cryst. 2010, 533, 102–112. [Google Scholar] [CrossRef]
- Saha, S.; Alam, R. Recent Developments in the Creation of a Single Molecular Sensing Tool for Ternary Iron (III), Chromium (III), Aluminium (III) Ionic Species: A Review. Luminescence 2023, 38, 1026–1046. [Google Scholar] [CrossRef]
- Suh, B.; Choe, D.; Kim, C. An Effective Colorimetric Sensor for Detecting Cu2+ Based on Benzothiazole Moiety. Color. Technol. 2021, 137, 512–519. [Google Scholar] [CrossRef]
- Erdemir, S.; Malkondu, S.; Alici, O. A Highly Selective and Sensitive Benzothiazole-based ‘Turn-on’ Fluorescent Sensor for Hg2+ Ion. Color. Technol. 2015, 131, 32–37. [Google Scholar] [CrossRef]
- Szala, M.; Nycz, J.E.; Malecki, G.J.; Sokolova, R.; Ramesova, S.; Switlicka-Olszewska, A.; Strzelczyk, R.; Podsiadly, R.; Machura, B. Synthesis of 5-Azo-8-Hydroxy-2-Methylquinoline Dyes and Relevant Spectroscopic, Electrochemical and Computational Studies. Dyes Pigment. 2017, 142, 277–292. [Google Scholar] [CrossRef]
- Arabahmadi, R.; Orojloo, M.; Amani, S. Azo Schiff Bases as Colorimetric and Fluorescent Sensors for Recognition of F−, Cd2+ and Hg2+ Ions. Anal. Methods 2014, 6, 7384–7393. [Google Scholar] [CrossRef]
- Orojloo, M.; Amani, S. A Highly Selective Chemosensor for Naked-Eye Detection of Fluoride and Aluminium(iii) Ions Based on a New Schiff Base Derivative. Aust. J. Chem. 2016, 69, 911–918. [Google Scholar] [CrossRef]
- Reena, V.; Suganya, S.; Velmathi, S. Synthesis and Anion Binding Studies of Azo-Schiff Bases: Selective Colorimetric Fluoride and Acetate Ion Sensors. J. Fluor. Chem. 2013, 153, 89–95. [Google Scholar] [CrossRef]
- Banerjee, S.; Brandão, P.; Saha, A. A Robust Fluorescent Chemosensor for Aluminium Ion Detection Based on a Schiff Base Ligand with an Azo Arm and Application in a Molecular Logic Gate. RSC Adv. 2016, 6, 101924–101936. [Google Scholar] [CrossRef]
- Mabhai, S.; Dolai, M.; Dey, S.K.; Choudhury, S.M.; Das, B.; Dey, S.; Jana, A.; Banerjee, D.R. A Naphthalene-Based Azo Armed Molecular Framework for Selective Sensing of Al3+. New J. Chem. 2022, 46, 6885–6898. [Google Scholar] [CrossRef]
- Abbaspour, A.; Esmaeilbeig, A.R.; Jarrahpour, A.A.; Khajeh, B.; Kia, R. Aluminium(III)-Selective Electrode Based on a Newly Synthesized Tetradentate Schiff Base. Talanta 2002, 58, 397–403. [Google Scholar] [CrossRef]
- Azadbakht, R.; Chidan, N.; Menati, S.; Koolivand, M. A New Azo-Schiff Base Dual-Mode Chemosensor: Colorimetric Detection of Cobalt Ions and Fluorometric Detection of Aluminum Ions in Aqueous Ethanol Solution. J. Fluoresc. 2023, 33, 527–538. [Google Scholar] [CrossRef]
- Mabhai, S.; Dolai, M.; Dey, S.K.; Dhara, A.; Choudhury, S.M.; Das, B.; Dey, S.; Jana, A. Rhodamine-Azobenzene Based Single Molecular Probe for Multiple Ions Sensing: Cu2+, Al3+, Cr3+ and Its Imaging in Human Lymphocyte Cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 219, 319–332. [Google Scholar] [CrossRef]
- Liao, X.; Fang, J.-A.; Zhao, J.-L.; Ruan, Q.; Zeng, X.; Luo, Q.-Y.; Redshaw, C. An Efficient ICT-Based Ratio/Colorimetric Tripodal Azobenzene Probe for the Recognition/Discrimination of F−, AcO− and H2PO4− Anions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 221, 117174. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Li, Q. Liquid Crystals: Versatile Self-Organized Smart Soft Materials. Chem. Rev. 2022, 122, 4887–4926. [Google Scholar] [CrossRef]
- Qaddoura, M.; Belfield, K. Synthesis, Characterization and Texture Observations of Calamitic Liquid Crystalline Compounds. Int. J. Mol. Sci. 2009, 10, 4772–4788. [Google Scholar] [CrossRef]
- Selvarasu, C.; Kannan, P. Effect of Azo and Ester Linkages on Rod Shaped Schiff Base Liquid Crystals and Their Photophysical Investigations. J. Mol. Struct. 2016, 1125, 234–240. [Google Scholar] [CrossRef]
- Sun, J.; Ge, Z.; Li, C.; Zhang, Z.; Wang, Y.; Jia, Y.; Tian, M.; Yao, D. Novel Branched Liquid Crystal Oligomers Containing Azo and Schiff Base Groups. J. Mol. Struct. 2023, 1273, 134322. [Google Scholar] [CrossRef]
- Katariya, K.D.; Nakum, K.J.; Hagar, M. New Fluorinated Azo/Schiff Base Liquid Crystals: Synthesis, Characterisation, Mesomorphic Study and DFT Calculations. Liq. Cryst. 2022, 49, 312–326. [Google Scholar] [CrossRef]
- Thaker, B.T.; Kanojiya, J.B.; Tandel, R.S. Effects of Different Terminal Substituents on the Mesomorphic Behavior of Some Azo-Schiff Base and Azo-Ester-Based Liquid Crystals. Mol. Cryst. Liq. Cryst. 2010, 528, 120–137. [Google Scholar] [CrossRef]
- Yeap, G.-Y.; Heng, B.-T.; Kakeya, M.; Takeuchi, D.; Gorecka, E.; Ito, M.M. Synthesis, 2D NMR and X-Ray Diffraction Studies on Cu(II) and Ni(II) Complexes with Ligands Derived from Azobenzene-Cored Schiff Base: Mesomorphic Behaviors of Cu(II)–Phenolates and Crystal Structure of Bis[4-(4-Alkoxy-2-Hydroxybenzylideneamino)Azobenzene]Copper(II). J. Mol. Struct. 2011, 999, 68–82. [Google Scholar] [CrossRef]
- Leech, D.; Kavanagh, P.; Schuhmann, W. Enzymatic Fuel Cells: Recent Progress. Electrochim. Acta 2012, 84, 223–234. [Google Scholar] [CrossRef]
- Gu, Y.; Yuan, L.; Jia, L.; Xue, P.; Yao, H. Recent Developments of a Co-Immobilized Laccase–Mediator System: A Review. RSC Adv. 2021, 11, 29498–29506. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, K.; Pradhan, S.; Haraguchi, T.; Sinha, C.; Parida, R.; Giri, S.; Roymahaptra, G.; Akitsu, T. Photo-Tunable Azobenzene-Anthraquinone Schiff Base Copper Complexes as Mediators for Laccase in Biofuel Cell Cathode. Symmetry 2020, 12, 797. [Google Scholar] [CrossRef]
- Kunitake, F.; Kim, J.-Y.; Yagi, S.; Yamzaki, S.; Haraguchi, T.; Akitsu, T. Chiral Recognition of Azo-Schiff Base Ligands, Their Cu(II) Complexes, and Their Docking to Laccase as Mediators. Symmetry 2019, 11, 666. [Google Scholar] [CrossRef]
- Kashiwagi, K.; Tassinari, F.; Haraguchi, T.; Banerjee-Gosh, K.; Akitsu, T.; Naaman, R. Electron Transfer via Helical Oligopeptide to Laccase Including Chiral Schiff Base Copper Mediators. Symmetry 2020, 12, 808. [Google Scholar] [CrossRef]
- Hou, A.; Zhang, C.; Wang, Y. Preparation and UV-Protective Properties of Functional Cellulose Fabrics Based on Reactive Azobenzene Schiff Base Derivative. Carbohydr. Polym. 2012, 87, 284–288. [Google Scholar] [CrossRef]
- Noser, A.A.; Ibrahim, S.A.; Abd El Salam, H.A.; El-Ebiary, N.M.A.; Mandour, H.S.A. Pyrazole-Vaniline Schiff Base Disperse Azo Dyes for UV Protective Clothing: Synthesis, Characterization, Comparative Study of UPF, Dyeing Properties and Potent Antimicrobial Activity. J. Iran. Chem. Soc. 2023, 20, 2963–2976. [Google Scholar] [CrossRef]
- Muthukrishnan, P.; Prakash, P.; Shankar, K.; Kathiresan, A. Azo Schiff Base as Antiscaling Agent for Mild Steel in Hydrochloric Acid: Electrochemical, Non-Electrochemical, and DFT Studies. J. Bio-Tribo-Corros. 2019, 5, 12. [Google Scholar] [CrossRef]
- Albo Hay Allah, M.A.; Balakit, A.A.; Salman, H.I.; Abdulridha, A.A.; Sert, Y. New Heterocyclic Compound as Carbon Steel Corrosion Inhibitor in 1 M H2SO4, High Efficiency at Low Concentration: Experimental and Theoretical Studies. J. Adhes. Sci. Technol. 2023, 37, 525–547. [Google Scholar] [CrossRef]
- Abdulridha, A.A.; Albo Hay Allah, M.A.; Makki, S.Q.; Sert, Y.; Salman, H.E.; Balakit, A.A. Corrosion Inhibition of Carbon Steel in 1 M H2SO4 Using New Azo Schiff Compound: Electrochemical, Gravimetric, Adsorption, Surface and DFT Studies. J. Mol. Liq. 2020, 315, 113690. [Google Scholar] [CrossRef]
- Yeğiner, G.; Gülcan, M.; Işık, S.; Ürüt, G.Ö.; Özdemir, S.; Kurtoğlu, M. Transition Metal (II) Complexes with a Novel Azo-Azomethine Schiff Base Ligand: Synthesis, Structural and Spectroscopic Characterization, Thermal Properties and Biological Applications. J. Fluoresc. 2017, 27, 2239–2251. [Google Scholar] [CrossRef]
- Kumar, Y.; Singh, N.K.; Singh, V.D.; Ali, I.; Tiwari, R.K.; Kumar, A.; Pandey, D.S. DNA/Protein Binding and Anticancer Activity of Zn(II) Complexes Based on Azo-Schiff Base Ligands. Inorg. Chim. Acta 2022, 538, 120963. [Google Scholar] [CrossRef]
- Mahdy, A.R.E.; Abu Ali, O.A.; Serag, W.M.; Fayad, E.; Elshaarawy, R.F.M.; Gad, E.M. Synthesis, Characterization, and Biological Activity of Co(II) and Zn(II) Complexes of Imidazoles-Based Azo-Functionalized Schiff Bases. J. Mol. Struct. 2022, 1259, 132726. [Google Scholar] [CrossRef]
- İspir, E. The Synthesis, Characterization, Electrochemical Character, Catalytic and Antimicrobial Activity of Novel, Azo-Containing Schiff Bases and Their Metal Complexes. Dyes Pigment. 2009, 82, 13–19. [Google Scholar] [CrossRef]
- Sarigul, M.; Deveci, P.; Kose, M.; Arslan, U.; Türk Dagi, H.; Kurtoglu, M. New Tridentate Azo–Azomethines and Their Copper(II) Complexes: Synthesis, Solvent Effect on Tautomerism, Electrochemical and Biological Studies. J. Mol. Struct. 2015, 1096, 64–73. [Google Scholar] [CrossRef]
- Kasare, M.S.; Dhavan, P.P.; Jadhav, B.L.; Pawar, S.D. Synthesis of Azo Schiff Base Ligands and Their Ni(II), Cu(II) and Zn(II) Metal Complexes as Highly-Active Antibacterial Agents. ChemistrySelect 2019, 4, 10792–10797. [Google Scholar] [CrossRef]
- Anitha, C.; Sumathi, S.; Tharmaraj, P.; Sheela, C.D. Synthesis, Characterization, and Biological Activity of Some Transition Metal Complexes Derived from Novel Hydrazone Azo Schiff Base Ligand. Int. J. Inorg. Chem. 2011, 2011, 493942. [Google Scholar] [CrossRef]
- Al-Hamdani, A.A.S.; Balkhi, A.M.; Falah, A.; Shaker, S.A. Synthesis and Investigation of Thermal Properties of Vanadyl Complexes with Azo-Containing Schiff-Base Dyes. J. Saudi Chem. Soc. 2016, 20, 487–501. [Google Scholar] [CrossRef]
- Sahoo, J.; Paidesetty, S.K. Biological Investigation of Novel Metal Complexes of 2-Amino-4-Substituted Phenylthiazole Schiff Bases. J. Taibah Univ. Med. Sci. 2018, 13, 142–155. [Google Scholar] [CrossRef]
- Al-Atbi, H.S.; Al-Salami, B.K.; Al-Assadi, I.J. New Azo-Azomethine Derivative of Sulfanilamide: Synthesis, Characterization, Spectroscopic, Antimicrobial and Antioxidant Activity Study. J. Phys. Conf. Ser. 2019, 1294, 052033. [Google Scholar] [CrossRef]
- Karem, L.; Ganim, F.; Al-Shemary, R. Synthesis, characterization, structural, thermal, pom studies, antimicrobial and DNA cleavage activity of a new schiff base-azo ligand and ITS complexation with selected metal ions. Biochem. Cell. Arch. 2018, 18, 1437–1448. [Google Scholar]
- Al Radi, K.A.; Fahad, T.A.; Ali, A.A. Synthesis and Spectral Characterization of Some Transition Metal Complexes of Azo-Schiff Base Derivative of Metoclopramide. 2018. [Google Scholar]
- Al Zoubi, W.; Al-Hamdani, A.A.S.; Ahmed, S.D.; Ko, Y.G. A New Azo-Schiff Base: Synthesis, Characterization, Biological Activity and Theoretical Studies of Its Complexes. Appl. Organomet. Chem. 2018, 32, e3895. [Google Scholar] [CrossRef]
- Selma, B. A Novel Azo-Schiff Base Ligand and Its Cobalt, Copper, Nickel Complexes: Synthesis, Characterization, Antimicrobial, Catalytic and Electrochemical Features. Anadolu Univ. J. Sci. Technol. A-Appl. Sci. Eng. 2016, 17, 315–326. [Google Scholar]


















| Klebsiella pneumoniae | Escherichia coli | Staphylococcus aureus | Pseudomonas aeruginosa | Enterococcus faecalis | Bacillus subtilis | Salmonella typhi | Reference | |
|---|---|---|---|---|---|---|---|---|
| UV2 a | 8 | 7 | 9 | 8 | - | - | - | [104] |
| Co(UV2)2 a | 7 | 7 | 8 | 7 | - | - | - | |
| Cu(UV2)2 a | 7 | 8 | 8 | 7 | - | - | - | |
| UV3(a) b | 64 | 64 | 16 | 64 | 16 | - | - | [105] |
| UV3(b) b | 64 | 64 | 16 | 64 | 8 | - | - | |
| CuUV3(a) b | 32 | 32 | 16 | 32 | 16 | - | - | |
| CuUV3(b) b | 32 | 32 | 16 | 32 | 16 | - | - | |
| UV4(a) a | <6 | <6 | <6 | <6 | - | <6 | - | [61] |
| UV4(b) a | <6 | <6 | 9–12 | <6 | - | >12 | - | |
| UV5(a) c | - | 250 | 32.25 | - | - | 62.5 | 125 | [106] |
| UV5(b) c | - | 250 | 3.90 | - | - | 125 | 250 | |
| UV5(c) c | - | 250 | 7.81 | - | - | 15.63 | 250 | |
| Zn(UV5(a))2 c | - | 62.5 | 7.81 | - | - | 7.81 | 125 | |
| Zn(UV5(b))2 c | - | 250 | 15.63 | - | - | 31.25 | 250 | |
| Zn(UV5(c))2 c | - | 62.5 | 3.90 | - | - | 7.81 | 125 | |
| UV6 a | - | 0 | 5–10 | 10 | - | 0 | - | [52] |
| Mn(UV6)2 a | - | 0 | 5–10 | 10–15 | - | 5–10 | - | |
| Ni(UV6)2 a | - | 0 | 0 | 10–15 | - | 0 | - | |
| Co(UV6)2 a | - | 10–15 | 10–15 | 10–15 | - | 0 | - | |
| Cu(UV6)2 a | - | 10–15 | 0 | 10–15 | - | 0 | - | |
| Zn(UV6)2 a | - | 0 | 0 | 20 | - | 0 | - | |
| Pd(UV6)OAc a | - | 15–20 | 10–15 | 10–15 | - | 10–15 | - | |
| UV7 b | - | 100 | 200 | 350 | - | 400 | - | [107] |
| Co(UV7) b | - | 15 | 10 | 25 | - | 20 | - | |
| Ni(UV7) b | - | 25 | 30 | 20 | - | 80 | - | |
| Cu(UV7) b | - | 25 | 10 | 20 | - | 15 | - | |
| Zn(UV7) b | - | 45 | 90 | 100 | - | 200 | - | |
| UV8a a | - | 19 | 17 | 16 | - | 20 | - | [108] |
| UV8b a | - | 22 | 20 | 18 | - | 22 | - | |
| VO(UV8a)SO4 a | - | 24 | 22 | 22 | - | 24 | - | |
| VO(UV8b)SO4 a | - | 16 | 20 | 21 | - | 20 | - | |
| Co(UV9a)2 a | 16.17 | 16.67 | 20.83 | - | - | - | - | [109] |
| Cu(UV9a)2 a | 16.00 | 15.83 | 18.33 | - | - | - | - | |
| Ni(UV9a)2 a | 14.67 | 0 | 14.83 | - | - | - | - | |
| Co(UV9b)2 a | 16.17 | 15.50 | 16.83 | - | - | - | - | |
| Cu(UV9b)2 a | 15.83 | 14.83 | 14.50 | - | - | - | - | |
| Ni(UV9b)2 a | 16.67 | 0 | 15.17 | - | - | - | - | |
| UV10a a | 20 | 21 | 20 | - | - | - | - | [110] |
| UV10b a | 19 | 18 | 18 | - | - | - | - | |
| UV10c a | 21 | 20 | 17 | - | - | - | - | |
| UV10d a | 20 | 49 | 45 | - | - | - | - | |
| UV10e a | 25 | 32 | 35 | - | - | - | - | |
| UV11 a | 9 | 6 | 12 | - | - | - | 15 | [111] |
| CoUV11 a | 9 | 18 | 16 | - | - | - | 18 | |
| NiUV11 a | 11 | 22 | 22 | - | - | - | 20 | |
| CuUV11 a | 17 | 27 | 19 | - | - | - | 20 | |
| CdUV11 a | 17 | 24 | 25 | - | - | - | 21 | |
| ZnUV11 a | 19 | 30 | 27 | - | - | - | 26 | |
| UV12 a | - | 21 | 23 | - | - | - | - | [112] |
| Ni(UV12)2 a | - | 21 | 17 | - | - | - | - | |
| UV13 a | - | 19 | 17 | 16 | - | 20 | - | [113] |
| Co(UV13)Cl a | - | 22 | 20 | 18 | - | 22 | - | |
| Ni(UV13)Cl a | - | 24 | 26 | 20 | - | 24 | - | |
| Pd(UV13)Cl a | - | 24 | 22 | 22 | - | 24 | - | |
| Pt(UV13)Cl3 a | - | 16 | 20 | 21 | - | 20 | - | |
| UV14 a | 9 | 10 | 13 | - | 0 | 12 | - | [114] |
| Cu(UV14)2 a | 12 | 12 | 20 | 0 | 15 | 17 | - | |
| Co(UV14)2 a | 9 | 11 | 9 | 0 | 0 | 11 | - | |
| Ni(UV14)2 a | 11 | 9 | 13 | 0 | 0 | 12 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonard, E.; Takeda, C.; Akitsu, T. Azobenzene-Containing Schiff-Bases—Syntheses and Dyes Applications. Colorants 2024, 3, 53-72. https://doi.org/10.3390/colorants3010004
Leonard E, Takeda C, Akitsu T. Azobenzene-Containing Schiff-Bases—Syntheses and Dyes Applications. Colorants. 2024; 3(1):53-72. https://doi.org/10.3390/colorants3010004
Chicago/Turabian StyleLeonard, Estelle, China Takeda, and Takashiro Akitsu. 2024. "Azobenzene-Containing Schiff-Bases—Syntheses and Dyes Applications" Colorants 3, no. 1: 53-72. https://doi.org/10.3390/colorants3010004