What’s the Madder? Characterization of Old Fashioned Alizarin/Aluminum Red Pigments Using Liquid and Solid-State NMR
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Alizarin Complexes and NMR Characterization

2.2. NMR Spectroscopy
2.3. Micro-Fourier Transform Infrared Spectroscopy
2.4. Mass Spectrometry
2.5. DFT Calculations
3. Results
3.1. Mass Spectrometry Characterization
3.2. Characterization of Alizarin Lake Pigments by Infrared Spectroscopy
3.3. Characterization of Alizarin Lake Pigments using Proton NMR Spectroscopy
3.3.1. Interconversion between Lake 1 and 2 as a Function of pH
3.3.2. Proton Diffusion Ordered Spectroscopy Experiments (1H-DOSY)
3.3.3. Prediction of Alizarin Lake Pigments NMR Chemical Shifts by Density Functional Theory
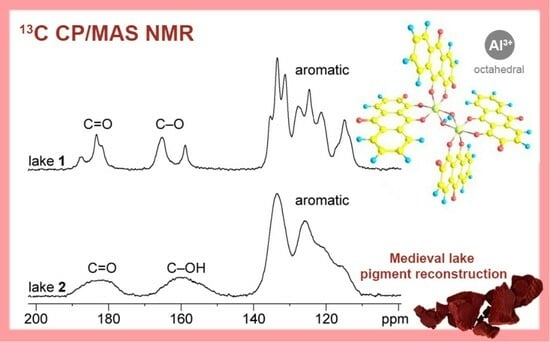
3.4. Characterization of Alizarin Lake Pigments using Solid-State (SS) NMR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuehni, R.G. Color: An Introduction to Practice and Principles, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2004. [Google Scholar]
- Paterson, I. A Dictionary of Colour; Thorogood Publishing Ltd.: London, UK, 2004. [Google Scholar]
- Pastoureau, M. Rouge. Histoire D’une Couleur; Éditions du Seuil: Paris, France, English version by Princeton University Press.
- Gage, J. Color in Art; Thames & Hudson: London, UK, 2006. [Google Scholar]
- Brusatin, M. Storia dei Colori; Einaudi: Torino, Italy, 1983. [Google Scholar]
- Cardon, D. Natural Dyes, Sources, Tradition, Technology and Science; Archetype Publications: London, UK, 2007. [Google Scholar]
- Chenciner, R. Madder Red—A History of Luxury and Trade; Curzon Press: London, UK, 2000. [Google Scholar]
- Veiga, T.; Moro, A.J.; Nabais, P.; Vilarigues, M.; Otero, V. A First Approach to the Study of Winsor & Newton’s 19th-Century Manufacture of Madder Red Lake Pigments. Heritage 2023, 6, 3606–3621. [Google Scholar]
- Shahid, M.; Wertz, J.; Degano, I.; Aceto, M.; Khan, M.I.; Quye, A. Analytical methods for determination of anthraquinone dyes in historical textiles: A review. Anal. Chim. Acta 2019, 1083, 58–87. [Google Scholar] [PubMed]
- Derksen, G.C.; Naayer, M.; van Beek, T.A.; Capelle, A.; Haaksman, I.K.; van Doren, H.A.; de Groot, Æ. Chemical and Enzymatic Hydrolysis of Anthraquinone Glycosides from Madder Roots. Phytochem. Anal. 2003, 14, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.J. History of Natural Dyes in the Ancient Mediterranean World. In Handbook of Natural Colorants; Bechtold, T., Mussak, R., Eds.; Wiley: Chichester, UK, 2009; pp. 3–20. [Google Scholar]
- Borgard, P.; Brun, J.-P.; Picon, M. L’Alun de Méditerranée; Publications du Centre Jean Bérard: Naples, Italy, 2005. [Google Scholar]
- Kirby, J. Paints, Pigments, Dyes. In Medieval Science, Technology and Medicine: An Encyclopedia; Glick, T., Livesey, S.J., Wallis, F., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 379–383. [Google Scholar]
- Innocenti, S.; Ricci, M.; Quintero Balbas, D.; Fontana, R.; Striova, J.; Becucci, M. Surface-enhanced Raman spectroscopy for madder lake detection in painting layers. Eur. Phys. J. Plus 2023, 138, 381. [Google Scholar] [CrossRef]
- Trigueiro, P.; Pereira, F.A.R.; Guillermin, D.; Rigaud, B.; Balme, S.; Janot, J.-M.; dos Santos, I.M.G.; Fonseca, M.G.; Walter, P.; Jaber, M. When anthraquinone dyes meet pillared montmorillonite: Stability or fading upon exposure to light? Dyes and Pigments 2018, 159, 384–394. [Google Scholar] [CrossRef]
- Zhuang, G.; Pedetti, S.; Bourlier, Y.; Jonnard, P.; Méthivier, C.; Walter, P.; Pradier, C.-M.; Jaber, M. New Insights into the Structure and Degradation of Alizarin Lake Pigments: Input of the Surface Study Approach. J. Phys. Chem. C 2020, 124, 12370–12380. [Google Scholar] [CrossRef]
- Brunello, F. L’Arte Della Tintura Nella Storia Dell Umanitá (The Art of Dyeing in the History of Mankind); Neri Pozza Editore: Vicenza, Italy, 1968. [Google Scholar]
- Grazia, C.; Clementi, C.; Miliani, C.; Romani, A. Photophysical properties of alizarin and purpurin Al(iii) complexes in solution and in solid state. Photochem. Photobiol. Sci. 2011, 10, 1249–1254. [Google Scholar] [CrossRef]
- Melo, M.J.; Claro, A. Bright light: Microspectrofluorimetry for the characterization of lake pigments and dyes in works of art. Acc. Chem. Res. 2010, 43, 857–866. [Google Scholar] [CrossRef]
- Claro, A.; Melo, M.J.; Schäfer, S.; de Melo, J.S.S.; Pina, F.; van den Berg, K.J.; Burnstock, A. The use of microspectrofluorimetry for the characterization of lake pigments. Talanta 2008, 74, 922–929. [Google Scholar] [CrossRef]
- Melo, M.J.; Nabais, P.; Vieira, M.; Araújo, R.; Otero, V.; Lopes, J.; Martín, L. Between past and future: Advanced studies of ancient colours to safeguard cultural heritage and new sustainable applications. Dyes and Pigments 2022, 208, 110815. [Google Scholar] [CrossRef]
- Parry, E.J.; Coste, J.H. The Chemistry of Pigments; Greenwood and Co.: London, UK, 1902. [Google Scholar]
- Beech, W.F.; Drew, D.K. Structure of the Aluminum Lakes of Some Azo-Dyes and Alizarin. J. Chem. Soc. 1940, 603–607. [Google Scholar] [CrossRef]
- Kiel, E.G.; Heertjes, P.M. Metal Complexes of Alizarin II—The Structure of Some Metal Complexes of Alizarin other than Turkey Red. J. Soc. Dye. Colour. 1963, 79, 61–64. [Google Scholar] [CrossRef]
- Kiel, E.G.; Heertjes, P.M. Metal Complexes of Alizarin I-The Structure of the Calcium-Aluminium Lake of Alizarin. J. Soc. Dye. Colour. 1963, 79, 21–27. [Google Scholar] [CrossRef]
- Wunderlich, C.; Bergerhoff, G. Konstitution und Farbe von Alizarin-und Purpurin-Farblacken. Chem. Ber. 1994, 127, 1185–1190. [Google Scholar] [CrossRef]
- Soubayrol, P.; Dana, G.; Man, P.P. Aluminium-27 solid-state NMR study of aluminium coordination complexes of Alizarin. Magn. Reson. Chem. 1996, 34, 638–645. [Google Scholar] [CrossRef]
- Sanyova, J. Spectroscopic Studies (Ftir, Sims, Es-Ms) on the Structure of Anthraquinone-Aluminium Complexes. In Dyes in History and Archaeology; Kirby, J., Ed.; Archetype Publications Ltd.: London, UK, 2008; pp. 208–213. [Google Scholar]
- Attree, G.F.; Perkin, A.G. Reduction products of the hydroxyanthraquinones. Part XII. J. Chem. Soc. Resumed 1931, 144–173. [Google Scholar] [CrossRef]
- Fierz-David, H.E.; Rutishauser, M. Die Zusammensetzung und Konstitution des Türkischrotes. Helv. Chim. Acta 1940, 23, 1298–1311. [Google Scholar] [CrossRef]
- Deb, B.K.; Ghosh, A.K. Chelated compounds and derivative of β-alkoxycarbonylalkyltin chlorides—5-arylazo-8-quinolinolates, alizarinates, and thiocyanate: Preparation and spectroscopic studies. Can. J. Chem. 1987, 65, 1241–1246. [Google Scholar] [CrossRef]
- DelMedico, A.; Pietro, W.J.; Lever, A.B.P. Linkage isomers of alizarin-bis(bipyridine)ruthenium(II). Inorg. Chim. Acta 1998, 281, 126–133. [Google Scholar] [CrossRef]
- DelMedico, A.; Fielder, S.S.; Lever, A.B.P.; Pietro, W.J. Rational Design of a Light-Driven Molecular Switch Incorporating an Alizarin-Ru(bpy)2 Fragment. Inorg. Chem. 1995, 34, 1507–1513. [Google Scholar] [CrossRef]
- Churchill, M.R.; Keil, K.M.; Bright, F.V.; Pandey, S.; Baker, G.A.; Keister, J.B. Linkage and Redox Isomerism in Ruthenium Complexes of Catecholate, Semiquinone, and o -Acylphenolate Ligands Derived from 1,2-Dihydroxy-9,10-anthracenedione (Alizarin) and Related Species: Syntheses, Characterizations, and Photophysics. Inorg. Chem. 2000, 39, 5807–5816. [Google Scholar] [CrossRef]
- Churchill, M.R.; Keil, K.M.; Gilmartin, B.P.; Schuster, J.J.; Keister, J.B.; Janik, T.S. Linkage and Redox Isomerism in Ruthenium Complexes of Catecholate, Semi-quinone, and o -Acylphenolate Ligands Derived from Tri- and Tetrahydroxy-9,10-anthracenediones. Inorg. Chem. 2001, 40, 4361–4367. [Google Scholar] [CrossRef] [PubMed]
- Zittel, H.E.; Florence, T.M. Voltammetric and spectrophotometric study of the zirconium-Alizarine Red S complex. Anal. Chem. 1967, 39, 320–326. [Google Scholar] [CrossRef]
- Jayaweera, P.M.; Jayarathne, T.A.U. Acid/base induced linkage isomerization of alizarin red adsorbed onto nano-porous TiO2 surfaces. Surf. Sci. 2006, 600, L297–L300. [Google Scholar] [CrossRef]
- Moriguchi, T.; Yano, K.; Nakagawa, S.; Kaji, F. Elucidation of adsorption mechanism of bone-staining agent alizarin red S on hydroxyapatite by FT-IR microspectroscopy. J. Colloid Interface Sci. 2003, 260, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Doskocz, M.; Kubas, K.; Frąckowiak, A.; Gancarz, R. NMR and ab initio studies of Mg2+, Ca2+, Zn2+, Cu2+ alizarin complexes. Polyhedron 2009, 28, 2201–2205. [Google Scholar] [CrossRef]
- 27Vitorino, T.; Melo, M.J.; Carlyle, L.; Otero, V. New insights into brazilwood lake pigments manufacture through the use of historically accurate reconstructions. Stud. Conserv. 2016, 61, 255–273. [Google Scholar] [CrossRef]
- Cañamares, M.V.; Garcia-Ramos, J.V.; Domingo, C.; Sanchez-Cortes, S. Surface-enhanced Raman scattering study of the adsorption of the anthraquinone pigment alizarin on Ag nanoparticles. J. Raman Spectrosc. 2004, 35, 921–927. [Google Scholar] [CrossRef]
- Hilbert, G.E.; Wulf, O.R.; Hendricks, S.B.; Liddel, U. The Hydrogen Bond between Oxygen Atoms in Some Organic Compounds. J. Am. Chem. Soc. 1936, 58, 548–555. [Google Scholar] [CrossRef]
- Bloom, H.; Briggs, L.H.; Cleverley, B. Physical properties of anthraquinone and its derivatives. Part I. Infrared spectra. J. Chem. Soc. Resumed 1959, 178–185. [Google Scholar] [CrossRef]
- Nakamoto, K.; McCarthy, P.J.; Ruby, A.; Martell, A.E. Infrared Spectra of Metal Chelate Compounds. II. Infrared Spectra of Acetylacetonates of Trivalent Metals. J. Am. Chem. Soc. 1961, 83, 1066–1069. [Google Scholar] [CrossRef]
- Nakamoto, K.; Martell, A.E. Infrared Spectra of Metal-Chelate Compounds. I. A Normal Coordinate Treatment on Bis-(Acetylacetonato)-Cu(II). J. Chem. Phys. 1960, 32, 588–597. [Google Scholar] [CrossRef]
- Mikami, M.; Nakagawa, I.; Shimanouchi, T. Far infra-red spectra and metal—Ligand force constants of acetylacetonates of transition metals. Spectrochim. Acta A 1960, 23, 1037–1053. [Google Scholar] [CrossRef]
- Fujita, J.; Martell, A.E.; Nakamoto, K. Infrared Spectra of Metal Chelate Compounds. VI. A Normal Coordinate Treatment of Oxalato Metal Complexes. J. Chem. Phys. 1962, 36, 324–331. [Google Scholar] [CrossRef]
- Holmgren, A.; Wu, L.; Forsling, W. Fourier transform infrared and Raman study of Alizarin Red S adsorbed at the fluorite–water interface. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1999, 55, 1721–1730. [Google Scholar] [CrossRef]
- REDiscover. Available online: https://www.instagram.com/rediscover_2023/ (accessed on 27 September 2023).
- Tissier, R.-C.; Rigaud, B.; Thureau, P.; Huix-Rotllant, M.; Jaber, M.; Ferré, N. Stressing the differences in alizarin and purpurin dyes through UV-visible light absorption and 1H-NMR spectroscopies. Phys. Chem. Chem. Phys. 2022, 24, 19452–19462. [Google Scholar] [CrossRef] [PubMed]









| Alizarin | Lake 1 | Lake 2 | IR Assignments * |
|---|---|---|---|
| ca. 3400 | ν(OH) | ||
| ca. 3400 | ca. 3400 | ν(OH) | |
| ca. 3000 | ca. 3200 | ca. 3200 | ν(CH) |
| 1662 | 1643 | 1657–1625 | ν(10-CO) + δ(CCC) |
| 1633 | ν(9-CO) | ||
| 1589 | 1591 | 1589 | ν(CC) |
| 1571 | 1571 | ν(CC) | |
| 1525 | 1529 | ν(CC) | |
| 1460 | 1467 | 1467 | ν(CO) + ν(CC) + δ(CH) |
| 1456 | 1454 | 1452 | ν(CC) + δ(2-COH) + δ(CH) |
| 1359 | 1358 | ν(CC) + δ(COH) | |
| 1350 | 1350 | ν(CC) + δ(1-COH) | |
| 1331 | 1331 | ν(CC) | |
| 1322 | ν(CC) | ||
| 1295 | 1288 | 1286 | ν(2-CO) + ν(CC) + δ(CCC) |
| 1267 | 1267 | 1265 | ν(CO) + ν(CC) |
| 1216 | 1209 | δ(CH) + δ(CCC) | |
| 1197 | ν(CC) + δ(CH) + δ(CCC) | ||
| 1182 | 1186 | 1190 | ν(CC) + δ(CH) + δ(CCC) |
| 1159 | 1151 | 1155 | ν(CC) + δ(CH) |
| Predicted Chemical Shift (ppm) † | ||||
|---|---|---|---|---|
| Lake 1 | Kiel and Heertjes Lake | Lake 2, 2m | Lake 2, 2M | |
| H3 | 4.89; 5.27 (5.08) | 6.66; 6.62 (6.64) | 6.82; 6.89 (6.86) | 5.97; 6.13 (6.05) |
| H4 | 7.05; 7.20 (7.12) | 7.75; 7.82 (7.78) | 7.71; 7.74 (7.72) | 6.61; 7.87 (7.24) |
| H5 | 7.61; 7.66 (7.64) | 8.28; 8.23 (8.26) | 8.31; 8.24 (8.28) | 8.15; 7.04 (7.60) |
| H6 | 6.66; 6.39 (6.52) | 7.65; 7.66 (7.66) | 7.68; 7.57 (7.62) | 7.61; 7.44 (7.52) |
| H7 | 7.69; 6.45 (7.07) | 7.51; 7.48 (7.50) | 7.58; 7.40 (7.49) | 7.55; 7.30 (7.42) |
| H8 | 9.77; 8.12 (8.94) | 8.45; 8.45 (8.45) | 8.23; 8.11 (8.17) | 8.52; 8.47 (8.50) |
| C1 | 166.0; 167.8 (166.9) | 159.5; 156.3 (157.9) | 151.7; 152.0 (151.8) | 163.7; 164.8 (164.2) |
| C2 | 169.8; 170.6 (170.2) | 161.8; 163.9 (162.8) | 149.0; 148.6 (148.8) | 173.4; 173.8 (173.6) |
| C3 | 109.6; 110.4 (110.0) | 119.7; 116.7 (118.2) | 111.4; 116.6 (114.0) | 120.2; 120.6 (120.4) |
| C9 | 170.6; 166.6 (168.8) | 180.3; 182.6 (181.4) | 178.3; 177.6 (178.0) | 168.7; 166.7 (167.7) |
| C10 | 162.1; 160.4 (161.2) | 169.1; 168.4 (168.8) | 172.6; 172.7 (172.6) | 142.0; 139.2 (140.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, L.C.; Otero, V.; Melo, M.J.; Cabrita, E.J.; Mafra, L. What’s the Madder? Characterization of Old Fashioned Alizarin/Aluminum Red Pigments Using Liquid and Solid-State NMR. Colorants 2023, 2, 601-617. https://doi.org/10.3390/colorants2040031
Silva LC, Otero V, Melo MJ, Cabrita EJ, Mafra L. What’s the Madder? Characterization of Old Fashioned Alizarin/Aluminum Red Pigments Using Liquid and Solid-State NMR. Colorants. 2023; 2(4):601-617. https://doi.org/10.3390/colorants2040031
Chicago/Turabian StyleSilva, Leonel C., Vanessa Otero, Maria J. Melo, Eurico J. Cabrita, and Luís Mafra. 2023. "What’s the Madder? Characterization of Old Fashioned Alizarin/Aluminum Red Pigments Using Liquid and Solid-State NMR" Colorants 2, no. 4: 601-617. https://doi.org/10.3390/colorants2040031