Metal-Free Counter Electrodes for DSSCs Based on Nitrogen-Doped Reduced Graphene Oxide Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of N-Doped Reduced Graphene Oxide Aerogels
2.2. Synthesis and Structural Characterization of the Sensitizer Dye
2.3. DSSC Device Fabrication
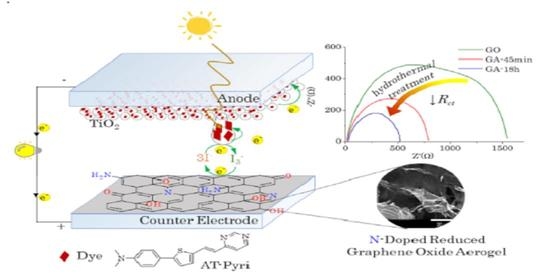
3. Results
3.1. Structural Characterization of the Graphene-Oxide-Based Materials
3.2. UV-Vis Transmittance Spectra and Electrochemical Characterization of Counter Electrodes
3.3. DSSCs Performance
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Yao, Z.; Guo, Y.; Wang, L.; Hao, Y.; Guo, Y.; Franchi, D.; Zhang, F.; Kloo, L.; Sun, L. Energy-Loss Reduction as a Strategy to Improve the Efficiency of Dye-Sensitized Solar Cells. Sol. RRL 2019, 3, 1900253. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, Y.; Wang, S.; Li, W.; Li, X.; Chen, J.; Wang, Z.-S.; Tian, H. Organic D-A-π-A Solar Cell Sensitizers with Improved Stability and Spectral Response. Adv. Funct. Mater. 2011, 21, 756–763. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, W.-H.; Zakeeruddin, S.M.; Grätzel, M. Insight into D–A−π–A Structured Sensitizers: A Promising Route to Highly Efficient and Stable Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 9307–9318. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Liao, X.; Gao, K.; Lin, F.; Xu, X.; Shi, X.; Zuo, L.; Liu, F.; Chen, Y.; Jen, A.K.-Y. Dithienopicenocarbazole-Based Acceptors for Efficient Organic Solar Cells with Optoelectronic Response Over 1000 nm and an Extremely Low Energy Loss. J. Am. Chem. Soc. 2018, 140, 2054–2057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cole, J.M. Anchoring Groups for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 3427–3455. [Google Scholar] [CrossRef]
- Delcamp, J.H.; Yella, A.; Nazeeruddin, M.K.; Grätzel, M. Modulating dye E(S+/S*) with efficient heterocyclic nitrogen containing acceptors for DSCs. Chem. Commun. 2012, 48, 2295–2297. [Google Scholar] [CrossRef]
- Reginato, G.; Calamante, M.; Zani, L.; Mordini, A.; Franchi, D. Design and synthesis of organic sensitizers with enhanced anchoring stability in dye-sensitized solar cells. Pure Appl. Chem. 2018, 90, 363–376. [Google Scholar] [CrossRef]
- Liang, M.; Chen, J. Arylamine organic dyes for dye-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 3453–3488. [Google Scholar] [CrossRef]
- Sivanadanam, J.; Ganesan, P.; Madhumitha, R.; Nazeeruddin, M.K.; Rajalingam, R. Effect of π-spacers on the photovoltaic properties of D–π–A based organic dyes. J. Photochem. Photobiol. A Chem. 2015, 299, 194–202. [Google Scholar] [CrossRef]
- Duerto, I.; Sarasa, S.; Barrios, D.; Orduna, J.; Villacampa, B.; Blesa, M.-J. Enhancing the temporal stability of DSSCs with novel vinylpyrimidine anchoring and accepting group. Dye. Pigm. 2022, 203, 110310. [Google Scholar] [CrossRef]
- Masud; Kim, H.K. Redox Shuttle-Based Electrolytes for Dye-Sensitized Solar Cells: Comprehensive Guidance, Recent Progress, and Future Perspective. ACS Omega 2023, 8, 6139–6163. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; He, B.; Kuang, P.; Yu, J.; Fan, K. NixSy Nanowalls/Nitrogen-Doped Graphene Foam Is an Efficient Trifunctional Catalyst for Unassisted Artificial Photosynthesis. Adv. Funct. Mater. 2018, 28, 1706917. [Google Scholar] [CrossRef]
- Ma, J.; Li, C.; Yu, F.; Chen, J. 3D Single-Walled Carbon Nanotube/Graphene Aerogels as Pt-Free Transparent Counter Electrodes for High Efficiency Dye-Sensitized Solar Cells. ChemSusChem 2014, 7, 3304–3311. [Google Scholar] [CrossRef]
- Carrera, C.; González-Domínguez, J.M.; Pascual, F.J.; Ansón-Casaos, A.; Benito, A.M.; Maser, W.K.; García-Bordejé, E. Modification of Physicochemical Properties and Boosting Electrical Conductivity of Reduced Graphene Oxide Aerogels by Postsynthesis Treatment. J. Phys. Chem. C 2020, 124, 13739. [Google Scholar] [CrossRef]
- Wu, M.; Lin, X.; Wang, Y.; Wang, L.; Guo, W.; Qi, D.; Peng, X.; Hagfeldt, A.; Grätzel, M.; Ma, T. Economical Pt-Free Catalysts for Counter Electrodes of Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2012, 134, 3419–3428. [Google Scholar] [CrossRef]
- Hou, S.; Cai, X.; Wu, H.; Yu, X.; Peng, M.; Yan, K.; Zou, D. Nitrogen-doped graphene for dye-sensitized solar cells and the role of nitrogen states in triiodide reduction. Energy Environ. Sci. 2013, 6, 3356–3362. [Google Scholar] [CrossRef]
- Sheng, Z.-H.; Shao, L.; Chen, J.-J.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H. Catalyst-Free Synthesis of Nitrogen-Doped Graphene via Thermal Annealing Graphite Oxide with Melamine and Its Excellent Electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef]
- Cui, Z.; Sheng, W. Thoughts about Choosing a Proper Counter Electrode. ACS Catal. 2023, 13, 2534–2541. [Google Scholar] [CrossRef]
- Rodríguez-Mata, V.; González-Domínguez, J.M.; Benito, A.M.; Maser, W.K.; García-Bordejé, E. Reduced Graphene Oxide Aerogels with Controlled Continuous Microchannels for Environmental Remediation. ACS Appl. Nano Mater. 2019, 2, 1210–1222. [Google Scholar] [CrossRef]
- González-Laínez, M.; Jiménez-Ruiz, M.T.; Martínez de Baroja, N.; Garín, J.; Orduna, J.; Villacampa, B.; Blesa, M.-J. Using functionalized nonlinear optical chromophores to prepare NLO-active polycarbonate films. Dye. Pigm. 2015, 119, 30–40. [Google Scholar] [CrossRef]
- García-Bordejé, E.; Víctor-Román, S.; Sanahuja-Parejo, O.; Benito, A.M.; Maser, W.K. Control of the Microstructure and Surface Chemistry of Graphene Aerogels via PH and Time Manipulation by a Hydrothermal Method. Nanoscale 2018, 10, 3526–3539. [Google Scholar] [CrossRef]
- Schwartz, V.; Fu, W.; Tsai, Y.-T.; Meyer, H.M.; Rondinone, A.J.; Chen, J.; Wu, Z.; Overbury, S.H.; Liang, C. Oxygen-Functionalized Few-Layer Graphene Sheets as Active Catalysts for Oxidative Dehydrogenation Reactions. ChemSusChem 2013, 6, 840–846. [Google Scholar] [CrossRef]
- Che, J.; Shen, L.; Xiao, Y. A new approach to fabricate graphene nanosheets in organic medium: Combination of reduction and dispersion. J. Mater. Chem. 2010, 20, 1722–1727. [Google Scholar] [CrossRef]
- Rodríguez-Mata, V.; Hernández-Ferrer, J.; Carrera, C.; Benito, A.M.; Maser, W.K.; García-Bordejé, E. Towards High-Efficient Microsupercapacitors Based on Reduced Graphene Oxide with Optimized Reduction Degree. Energy Storage Mater. 2020, 25, 740–749. [Google Scholar] [CrossRef]
- Duerto, I.; García-Palacín, M.; Barrios, D.; Garín, J.; Orduna, J.; Villacampa, B.; Blesa, M.-J. A novel σ-linkage to dianchor dyes for efficient dyes sensitized solar cells: 3-methyl-1,1-cyclohexane. Dye. Pigm. 2020, 173, 107945. [Google Scholar] [CrossRef]
- Laschuk, N.O.; Easton, E.B.; Zenkina, O.V. Reducing the resistance for the use of electrochemical impedance spectroscopy analysis in materials chemistry. RSC Adv. 2021, 11, 27925–27936. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, G.-D.; Liu, Y.; Yang, L.; Lian, X.; Asefa, T.; Zou, X. Overall Water Splitting Catalyzed Efficiently by an Ultrathin Nanosheet-Built, Hollow Ni3S2-Based Electrocatalyst. Adv. Funct. Mater. 2016, 26, 4839–4847. [Google Scholar] [CrossRef]
- Muñoz-García, A.B.; Benesperi, I.; Boschloo, G.; Concepcion, J.J.; Delcamp, J.H.; Gibson, E.A.; Meyer, G.J.; Pavone, M.; Pettersson, H.; Hagfeldt, A.; et al. Dye-sensitized solar cells strike back. Chem. Soc. Rev. 2021, 50, 12450–12550. [Google Scholar] [CrossRef] [PubMed]
- Zambrzycki, M.; Piech, R.; Ruiz Raga, S.; Lira-Cantu, M.; Fraczek-Szczypta, A. Hierarchical carbon nanofibers/carbon nanotubes/NiCo nanocomposites as novel highly effective counter electrode for dye-sensitized solar cells: A structure-electrocatalytic activity relationship study. Carbon 2023, 203, 97–110. [Google Scholar] [CrossRef]
- Xi, J.-Y.; Jia, R.; Li, W.; Wang, J.; Bai, F.-Q.; Eglitis, R.I.; Zhang, H.-X. How does graphene enhance the photoelectric conversion efficiency of dye sensitized solar cells? An insight from a theoretical perspective. J. Mater. Chem. A 2019, 7, 2730–2740. [Google Scholar] [CrossRef]
- Yang, R.-Y.; Chen, H.-Y.; Lai, F.-D. Performance Degradation of Dye-Sensitized Solar Cells Induced by Electrolytes. Adv. Mater. Sci. Eng. 2012, 2012, 902146. [Google Scholar] [CrossRef] [Green Version]
- Lai, S.C.; Connor, K.N.P.; Ke, L. Degradation Mechanisms and Electron Kinetics Analysis in Aging Dye Sensitized Solar Cell Using Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2011, 158, H1193. [Google Scholar] [CrossRef]




| Elemental Analysis | XPS | |||
|---|---|---|---|---|
| O/C | N/C | O/C | N/C | |
| wt% | wt% | wt% | wt% | |
| 72.4 | 4.1 | n.d | n.d. | GO |
| 14.9 | 10.6 | 8.9 | 6.5 | GA-45 min |
| 13.0 | 10.3 | 8.0 | 6.3 | GA-18 h |
| η (%) | ff (%) | J SC (mA cm−2) | VOC (V) | Catalyst |
|---|---|---|---|---|
| 1.0 | 18 | 8.88 | 0.620 | GO |
| 1.7 | 32 | 9.00 | 0.590 | GA-45 min |
| 1.2 | 35 | 7.69 | 0.445 | GA-18 h |
| 2.7 | 67 | 7.23 | 0.560 | Pt |
| η (%) | ff (%) | JSC (mA cm−2) | VOC (V) | Catalyst |
|---|---|---|---|---|
| 1.1 | 23 | 7.76 | 0.605 | GO |
| 1.8 | 39 | 7.98 | 0.575 | GA-45 min |
| 1.4 | 45 | 6.57 | 0.470 | GA-18 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duerto, I.; Carrera, C.; Barrios, D.; Benito, A.M.; Maser, W.K.; Villacampa, B.; García-Bordejé, E.; Blesa, M.-J. Metal-Free Counter Electrodes for DSSCs Based on Nitrogen-Doped Reduced Graphene Oxide Materials. Colorants 2023, 2, 443-452. https://doi.org/10.3390/colorants2020020
Duerto I, Carrera C, Barrios D, Benito AM, Maser WK, Villacampa B, García-Bordejé E, Blesa M-J. Metal-Free Counter Electrodes for DSSCs Based on Nitrogen-Doped Reduced Graphene Oxide Materials. Colorants. 2023; 2(2):443-452. https://doi.org/10.3390/colorants2020020
Chicago/Turabian StyleDuerto, Isolda, Clara Carrera, Daniel Barrios, Ana M. Benito, Wolfgang K. Maser, Belén Villacampa, Enrique García-Bordejé, and María-Jesús Blesa. 2023. "Metal-Free Counter Electrodes for DSSCs Based on Nitrogen-Doped Reduced Graphene Oxide Materials" Colorants 2, no. 2: 443-452. https://doi.org/10.3390/colorants2020020