Photocatalytic Properties of PbMoO4 Nanocrystals against Cationic and Anionic Dyes in Several Experimental Conditions
Abstract
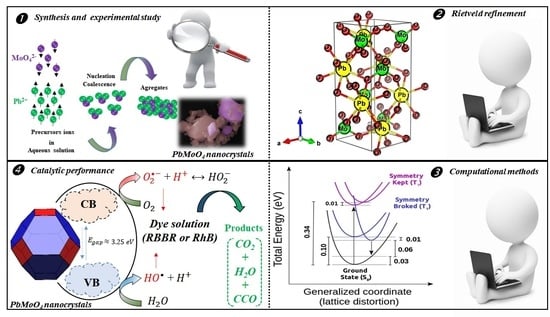
:1. Introduction
2. Materials and Methods
2.1. Synthesis of PbMoO4 Nanocrystals
2.2. Analytical Methods
2.2.1. XRD Measurement and Rietveld Refinement of PbMoO4 Nanocrystals
2.2.2. Vibrational Raman Spectroscopy
2.2.3. UV–Vis by Diffuse Spectroscopy (DRS)
2.2.4. Field Emission Scanning Electron Microscopy—FE-SEM
2.2.5. Photocatalytic Performance of PbMoO4 Nanocrystals under Degradation of Cationic and Anionic Dye
2.3. Computational Methods for PbMoO4 Nanocrystals
3. Results and Discussion
3.1. XRD Pattern and Rietveld Refinement
3.2. Micro-Raman Vibrational Spectroscopy
3.3. UV–Vis Spectroscopy by Diffuse Reflectance
3.4. Atomic Structure and Electronic States
3.5. FE-SEM Images of PbMoO4 Nanocrystals
3.6. Photocatalysis of RhB and RBBR Dyes with PbMoO4 Nanocrystals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gyawali, G.; Adhikari, R.; Joshi, B.; Kim, T.H.; Rodríguez-González, V.; Lee, S.W. Sonochemical synthesis of solar-light-driven Ag-PbMoO4 photocatalyst. J. Hazard. Mater. 2013, 263, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Nobre, F.X.; Mendes, O.C.; da Silva, A.P.J.; Junior, J.L.S.; Nascimento, M.V.B.; Junior, W.A.G.P.; Manzato, L.; Brandim, A.S.; Matos, J.M.E.; Brito, W.R.; et al. Fast and ef-ficient green synthesis of CaWO4 NPs using eggshells as a biogenic calcium source: Structure, optical property, and morphology. J. Photochem. Photobiol. A Chem. 2023, 439, 114589. [Google Scholar] [CrossRef]
- Hernández-Uresti, D.B.; Sanchez-Martinez, D.; Torres-Martinez, L.M. Novel visible light-driven PbMoO4/g-C3N4hybrid composite with enhanced photocatalytic performance. J. Photochem. Photobiol. A Chem. 2017, 345, 21–26. [Google Scholar] [CrossRef]
- Gomes, E.O.; Gracia, L.; Santiago, A.A.G.; Tranquilin, R.L.; Motta, F.V.; Amoresi, R.A.C.; Longo, E.; Bomio, M.R.D.; Andres, J. Structure, electronic properties, morphology evolution, and photocata-lytic activity in PbMoO4 and Pb1-2: XCaxSrxMoO4(x = 0.1, 0.2, 0.3, 0.4 and 0.5) solid solutions. Phys. Chem. Chem. Phys. 2020, 22, 25876–25891. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Aryal, P.; Kim, H.; Lee, M.H.; Kim, Y. PbMoO4 Synthesis from Ancient Lead and Its Single Crystal Growth for Neutrinoless Double Beta Decay Search. Crystals 2020, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Moura, J.V.B.; Freitas, T.S.; Silva, A.R.P.; Santos, A.T.L.; da Silva, J.H.; Cruz, R.P.; Pereira, R.L.S.; Freire, P.T.C.; Luz-Lima, C.; Pinheiro, G.S.; et al. Synthesis, characterizations, and antibacterial properties of PbMoO4 nanocrystals. Arab. J. Chem. 2018, 11, 739–746. [Google Scholar] [CrossRef]
- Isik, M.; Gasanly, N.M.; Darvishov, N.H.; Bagiev, V.E. Structural and temperature-tuned band gap energy characteristics of PbMoO4 single crystals. Opt. Mater. 2022, 126, 112210. [Google Scholar] [CrossRef]
- Rajkumar, M.; Arunpandian, M.; Leeladevi, K.; Rameshkumar, P.; Arunachalam, S. Fabrication of pebble stone-like PbMoO4 nanostructure: Focus on photocatalysis, photoluminescence and electron density distribution analysis. Phys. B Condens. Matter. 2021, 620, 413222. [Google Scholar] [CrossRef]
- Bomio, M.R.D.; Tranquilin, R.L.; Motta, F.V.; Paskocimas, C.A.; Nascimento, R.M.; Gracia, L.; Andres, J.; Longo, E. Toward understanding the photocatalytic activity of PbMoO4 powders with predominant (111), (100), (011), and (110) facets. A combined experimental and theoretical study. J. Phys. Chem. C 2013, 117, 21382–21395. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Yi, H.; Ke, Q.; Zhang, J. Preparation of a AgCl/PbMoO4 Composite and Investigation of Its Photocatalytic Oxidative Desulfurization Performance. ACS Omega 2020, 5, 10927–10938. [Google Scholar] [CrossRef]
- Martínez-de la Cruz, A.; Hernández-Uresti, D.B.; Torres-Martínez, L.M.; Lee, S.W. Photocatalytic properties of PbMoO4 synthesized by a hydrothermal reaction. React. Kinet. Mech. Catal. 2012, 107, 467–475. [Google Scholar] [CrossRef]
- Hernández-Uresti, D.B.; Martínez-De La Cruz, A.; Aguilar-Garib, J.A. Photocatalytic activity of PbMoO4 molybdate synthesized by microwave method. Catal. Today 2013, 212, 70–74. [Google Scholar] [CrossRef]
- Hernández-Uresti, D.B.; Martínez-de la Cruz, A.; Torres-Martínez, L.M. Photocatalytic degradation of organic compounds by PbMoO4 synthesized by a microwave-assisted solvothermal method. Ceram. Int. 2016, 42, 3096–3103. [Google Scholar] [CrossRef]
- Gurgel, G.M.; Lovisa, L.X.; Conceição, O.L.A.; Li, M.S.; Longo, E.; Paskocimas, C.A.; Motta, F.V.; Bomio, M.R.D. Evaluation of morphology and photoluminescent properties of PbMoO4 crystals by ultrasonic amplitude. J. Mater. Sci. 2017, 52, 4608–4620. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Wu, Q. Fabrication of carbon microspheres@PbMoO4 core-shell hybrid structures and its visible light-induced photocatalytic activity. Catal. Commun. 2012, 24, 85–89. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Y.; Chen, D.; Bao, F. Evolution of single crystalline dendrites from nanoparticles through oriented attachment. J. Phys. Chem. B 2005, 109, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Hashim, M.; Hu, C.; Wang, X.; Li, X.; Guo, D. Synthesis and photocatalytic property of lead molybdate dendrites with exposed (0 0 1) facet. Appl. Surf. Sci. 2012, 258, 5858–5862. [Google Scholar] [CrossRef]
- Dai, K.; Yao, Y.; Liu, H.; Mohamed, I.; Chen, H.; Huang, Q. Enhancing the photocatalytic activity of lead molybdate by modifying with fullerene. J. Mol. Catal. A Chem. 2013, 374–375, 111–117. [Google Scholar] [CrossRef]
- Rodríquez-Carvajal, J. Magnetic structure determination from powder diffraction symmetry analysis and simulated annealing. Mater. Sci. Forum 2001, 378–381, 268–273. [Google Scholar] [CrossRef]
- Lugli, C.; Medici, L.; Saccardo, D. Natural wulfenite: Structural refinement by single-crystal X-ray diffraction. Neues Jahrb. Für. Mineral. 1999, 6, 281–288. [Google Scholar]
- Du, W.; Liu, L.; Zhou, K.; Ma, X.; Hao, Y.; Qian, X. Black lead molybdate nanoparticles: Facile synthesis and photocatalytic properties responding to visible light. Appl. Surf. Sci. 2015, 328, 428–435. [Google Scholar] [CrossRef]
- Nobre, F.X.; Bastos, I.S.; dos Santos Fontenelle, R.O.; Júnior, E.A.A.; Takeno, M.L.; Manzato, L.; de Matos, J.M.E.; Orlandi, P.P.; Mendes, J.D.F.S.; Brito, W.R.; et al. Antimicrobial properties of α-Ag2WO4 rod-like microcrystals synthesized by sonochemistry and sonochemistry followed by hydrothermal conventional method. Ultrason. Sonochem. 2019, 58, 104620. [Google Scholar] [CrossRef]
- Batista, C.Y.P.; Romaguera–Barcelay, Y.; Matos, R.S.; Pedraça, A.D.S.A.; do Amaral Amâncio, M.; Kourouma, A.; Ruiz, Y.L.; Cotta, E.A.; Brito, W.R.; Gandarilla, A.M.D. Morphology, microstructure, and electrocatalytical properties of sol-gel spin-coated Bi0.5Na0.5Ba(TiO3)2 thin films. Appl. Surf. Sci. 2023, 615, 156374. [Google Scholar] [CrossRef]
- Silva, M.D.P.; Gonçalves, R.F.; Nogueira, I.C.; Longo, V.M.; Mondoni, L.; Moron, M.G.; Santana, Y.V.; Longo, E. Microwave-assisted hydrothermal synthesis of Ag2(W1-xMox)O4 heterostructures: Nucleation of Ag, morphology, and photoluminescence properties. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2016, 153, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Leciejewicz, J. A neutron crystallographic investigation of lead molybdenum oxide, PbMoO4. Zeitschrift für Krist. 1965, 121, 158–164. [Google Scholar] [CrossRef]
- Dovesi, R.; Erba, A.; Orlando, R.; Zicovich-Wilson, C.M.; Civalleri, B.; Maschio, L.; Rérat, M.; Casassa, S.; Baima, J.; Salustro, S.; et al. Quantum-mechanical condensed matter simulations with CRYSTAL. Wiley Comput. Mol. Sci. 2018, 8, e1360. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef] [Green Version]
- Lecklider, T. Maintainng a heathy rhythm. EE-Eval. Eng. 2011, 50, 36–39. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, A.R.; Bruix, A.; dos Santos, I.M.; Sambrano, J.R.; Illas, F. DFT Study on Ce-Doped Anatase TiO2: Nature of Ce3+ and Ti3+ Centers Triggered by Oxygen Vacancy Formation. J. Phys. Chem. C. 2014, 118, 9677–9689. [Google Scholar] [CrossRef]
- Albuquerque, A.R.; Bruix, A.; Sambrano, J.R.; Illas, F. Theoretical Study of the Stoichiometric and Reduced Ce-Doped TiO2 Anatase (001) Surfaces. J. Phys. Chem. C. 2015, 119, 4805–4816. [Google Scholar] [CrossRef]
- Ferrero, M.; Rérat, M.; Kirtman, B.; Dovesi, R. Calculation of first and second static hyperpolarizabilities of one- to three-dimensional periodic compounds. Implementation in the <scp>CRYSTAL</scp> code. J. Chem. Phys. 2008, 129, 244110. [Google Scholar] [CrossRef]
- Crystal Base Sets. 2022. Available online: https://www.crystal.unito.it/basis_sets.html (accessed on 12 July 2022).
- Prosandeev, S.A.; Waghmare, U.; Levin, I.; Maslar, J. First-order Raman spectra of double perovskites AB′ 1/2B′′1/2O3. Phys. Rev. B 2005, 71, 214307. [Google Scholar] [CrossRef] [Green Version]
- Canepa, P.; Hanson, R.M.; Ugliengo, P.; Alfredsson, M. J-ICE: A new Jmol interface for handling and visualizing crystallographic and electronic properties. J. Appl. Crystallogr. 2011, 44, 225–229. [Google Scholar] [CrossRef]
- Kokalj, A. Computer graphics and graphical user interfaces as tools in simulations of matter at the atomic scale. Comput. Mater. Sci. 2003, 28, 155–168. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Nassif, V.; Carbonio, R.E.; Alonso, J.A. Neutron Diffraction Study of the Crystal Structure of BaMoO4: A Suitable Precursor for Metallic BaMoO3 Perovskite. J. Solid State Chem. 1999, 146, 266–270. [Google Scholar] [CrossRef]
- Anitha, T.; Reddy, A.E.; Kumar, Y.A.; Cho, Y.-R.; Kim, H.-J. One-step synthesis and electrochemical performance of a PbMoO4/CdMoO4 composite as an electrode material for high-performance supercapacitor applications. Dalt. Trans. 2019, 48, 10652–10660. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Andreeva, O.P.; Korolkov, I.V.; Maximovskiy, E.A.; Lim, C.S. Low-Temperature Synthesis and Structural Properties of PbMoO4 Nanocrystals. Asian J. Chem. 2014, 26, 1287–1289. [Google Scholar] [CrossRef]
- Souza, E.L.S.; Sczancoski, J.C.; Nogueira, I.C.; Almeida, M.A.P.; Orlandi, M.O.; Li, M.S.; Luz, R.A.S.; Filho, M.G.R.; Longo, E.; Cavalcante, L.S. Structural evolution, growth mechanism and photoluminescence properties of CuWO4 nanocrystals. Ultrason. Sonochem. 2017, 38, 256–270. [Google Scholar] [CrossRef] [Green Version]
- Cavalcante, L.S.; Sczancoski, J.C.; Batista, N.C.; Longo, E.; Varela, J.A.; Orlandi, M.O. Growth mechanism and photocatalytic properties of SrWO4 microcrystals synthesized by injection of ions into a hot aqueous solution. Adv. Powder Technol. 2013, 4, 344–353. [Google Scholar] [CrossRef]
- Melo, A.D.; da Silva, J.P.; Nobre, F.X.; Costa, S.; Sales, J.C.C.; Anglada-Rivera, J.; Guerrero, F.; da, M.M.; de Souza, R.F.B.; Peña-Garcia, R.; et al. Synthesis microwave-assisted: Fast method to obtain lithium-doped sodium titanate. J. Mater. Sci. Mater. Electron. 2023, 34. [Google Scholar] [CrossRef]
- Longo, E.; Volanti, D.P.; Longo, V.M.; Gracia, L.; Nogueira, I.C.; Almeida, M.A.P.; Pinheiro, A.N.; Ferrer, M.M.; Cavalcante, L.S.; Andrés, J. Toward an understanding of the growth of Ag filaments on α-Ag2WO4 and their photoluminescent properties: A combined experimental and theoretical study. J. Phys. Chem. C 2014, 118, 1229–1239. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, D.; Zhou, M.; Pan, C. Surfactant-free hydrothermal synthesis, growth mechanism and photocatalytic properties of PbMoO4 polyhedron microcrystals. J. Saudi Chem. Soc. 2017, 21, S275–S282. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Vidya, S.; Thomas, J.K. Study on the optical band gap and photoluminescence of PbMoO4 nano powder synthesized by an auto igniting combustion technique. IOP Conf. Ser. Mater. Sci. Eng. 2015, 73, 012120. [Google Scholar] [CrossRef] [Green Version]
- Bindu, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.G.; Hyeon, T. Formation mechanisms of uniform nanocrystals via hot-injection and heat-up methods. Small 2011, 7, 2685–2702. [Google Scholar] [CrossRef]
- da Silva Sousa, G.; Xavier Nobre, F.; Valério Botelho do Nascimento, M.; da Cunha Mendes, O.; Manzato, L.; Leyet Ruiz, Y.; Brito, W.R.; Rogério da Costa Couceiro, P.; Elias de Matos, J.M. Rietveld Refinement, Morphology, and Optical and Photoluminescence Properties of a β-Ag1.94Cu0.06MoO4 Solid Solution. Inorg. Chem. 2022, 61, 1530–1537. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. An article on optics of paint layers. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- de Santana, Y.V.B.; Gomes, J.E.C.; Matos, L.; Cruvinel, G.H.; Perrin, A.; Perrin, C.; Andrès, J.; Varela, J.A.; Longo, E. Silver molybdate and silver tungstate nanocomposites with enhanced photoluminescence. Nanomater. Nanotechnol. 2014, 4, 1–10. [Google Scholar] [CrossRef]
- Cavalcante, L.S.; Almeida, M.A.P.; Avansi, W.; Tranquilin, R.L.; Longo, E.; Batista, N.C.; Mastelaro, V.R.; Li, M.S. Cluster coordination and photoluminescence properties of α-Ag2WO4 microcrystals. Inorg. Chem. 2012, 51, 10675–10687. [Google Scholar] [CrossRef] [PubMed]
- Pinatti, I.M.; Nogueira, I.C.; Pereira, W.S.; Pereira, P.F.S.; Gonçalves, R.F.; Varela, J.A.; Longo, E.; Rosa, I.L.V. Structural and photoluminescence properties of Eu3+ doped α-Ag2WO4 synthesized by the green coprecipitation methodology. Dalt. Trans. 2015, 44, 17673–17685. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, M.T.; Gracia, L.; Silva, G.S.; Santos, L.P.S.; Andrés, J.; Cordoncillo, E.; Longo, E. Understanding the formation and growth of Ag nanoparticles on silver chromate induced by electron irradiation in electron microscope: A combined experimental and theoretical study. J. Solid State Chem. 2016, 239, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Roca, R.A.; Sczancoski, J.C.; Nogueira, I.C.; Fabbro, M.T.; Alves, H.C.; Gracia, L.; Santos, L.P.S.; de Sousa, C.P.; Andrés, J.; Luz, G.E.; et al. Facet-dependent photocatalytic and antibacterial properties of α-Ag2WO4 crystals: Combining experimental data and theoretical insights. Catal. Sci. Technol. 2015, 5, 4091–4107. [Google Scholar] [CrossRef] [Green Version]
- Basilio, L.A.L.; Silva, J.H.L.; Xavier, F.; Aguilera, L.; Guerrero, F.; Antonelli, E.; Anglada-Rivera, J.; Garcia, J.E.; Silva, R.S.; Leyet, Y. Toward a new PTCR material based on the Na2Ti6O13/Na2Ti3O7 system. Mater. Res. Bull. 2021, 140, 111311. [Google Scholar] [CrossRef]
- Bi, J.; Wu, L.; Zhang, Y.; Li, Z.; Li, J.; Fu, X. Solvothermal preparation, electronic structure and photocatalytic properties of PbMoO4 and SrMoO4. Appl. Catal. B Environ. 2009, 91, 135–143. [Google Scholar] [CrossRef]
- Rellán-Piñeiro, M.; López, N. One Oxygen Vacancy, Two Charge States: Characterization of Reduced α-MoO3(010) through Theoretical Methods. J. Phys. Chem. Lett. 2018, 9, 2568–2573. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Jin, X.; Shi, J.; Xie, H.; Xu, H.; Zhang, T.; Wang, Y.; Li, C. Boosting the Photocarrier Separation of PbMoO4 through Facet Collaboration. ACS Appl. Energy Mater. 2021, 4, 14287–14294. [Google Scholar] [CrossRef]
- Lim, C.S.; Aleksandrovsky, A.S.; Atuchin, V.V.; Molokeev, M.S.; Oreshonkov, A.S. Microwave sol-gel synthesis, microstructural and spectroscopic properties of scheelite-type ternary molybdate upconversion phosphor NaPbLa(MoO4)3:Er3+/Yb3+. J. Alloys Compd. 2020, 826, 152095. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zhu, R.; Zhu, G.; Wang, M.; Chen, Y.; Zhu, J.; Xi, Y.; He, H. Plasmonic Ag coated Zn/Ti-LDH with excellent photocatalytic activity. Appl. Surf. Sci. 2018, 433, 458–467. [Google Scholar] [CrossRef] [Green Version]
- Özçimen, D.; Salan, T. Uklanjanje reaktivne boje remazol brilliant blue R IZ vodenih rastvora adsorbentom NA bazi otpadnog mulja IZ anaerobne digestije. Chem. Ind. Chem. Eng. Q. 2016, 22, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Yetim, T.; Tekin, T. A Kinetic Study on Photocatalytic and Sonophotocatalytic Degradation of Textile Dyes. Period. Polytech. Chem. Eng. 2016, 61, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Postai, D.L.; Demarchi, C.A.; Zanatta, F.; Melo, D.C.C.; Rodrigues, C.A. Adsorption of rhodamine B and methylene blue dyes using waste of seeds of Aleurites Moluccana, a low cost adsorbent. Alexandria Eng. J. 2016, 55, 1713–1723. [Google Scholar] [CrossRef] [Green Version]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Gold nanoparticles-sensitized wide and narrow band gap TiO2 for visible light applications: A comparative study. New J. Chem. 2015, 39, 4708–4715. [Google Scholar] [CrossRef]











| Method | Time (min) | Temp. (°C) | Lattices Parameters | V ) | Ref | |
|---|---|---|---|---|---|---|
| MH | 1 | 25 | 5.433(2) | 12.103(9) | 357.30 | This work |
| MH | 10 | 25 | 5.433(2) | 12.103(7) | 357.30 | This work |
| MH | 30 | 25 | 5.433(3) | 12.102(7) | 357.29 | This work |
| MH | 60 | 25 | 5.433(3) | 12.102(7) | 357.28 | This work |
| HC | 120 | 150 | 5.4350 | 12.1066 | 357.63 | [6] |
| HC | 1200 | 160 | 5.4336 | 12.1104 | 357.456 | [16] |
| SR | 720 | 1150 | 5.4351 | 12.1056 | 357.60 | [38] |
| ICSD n°. 26784 | - | 5.4312 | 12.1065 | 357.12(24) | - | |
| Sample | RhB Dye | RBBR Dye | ||||
|---|---|---|---|---|---|---|
| Disc. (%) | (min−1) | (min) | Disc. (%) | (min−1) | (min) | |
| Photolysis | 16.38(7) | 1.88 | 368.6 | 6.27(5) | 0.364 | 1904.2 |
| PbMoO4-1 | 100 | 26.77 | 25.8 | 82.56(5) | 8.64 | 80.2 |
| PbMoO4-10 | 100 | 52.45 | 13.2 | 100 | 18.87 | 36.7 |
| PbMoO4-30 | 81.99(5) | 13.41 | 51.6 | 93.18(4) | 9.06 | 76.5 |
| PbMoO4-60 | 100 | 13.38 | 51.8 | 76.0 | 7.72 | 89.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nobre, F.; Trindade, J.; do Nascimento, M.; Souza, G.; Mendes, O.; Albuquerque, A.; Sambrano, J.; Couceiro, P.; Brito, W.; Ruiz, Y.L.; et al. Photocatalytic Properties of PbMoO4 Nanocrystals against Cationic and Anionic Dyes in Several Experimental Conditions. Colorants 2023, 2, 111-134. https://doi.org/10.3390/colorants2010008
Nobre F, Trindade J, do Nascimento M, Souza G, Mendes O, Albuquerque A, Sambrano J, Couceiro P, Brito W, Ruiz YL, et al. Photocatalytic Properties of PbMoO4 Nanocrystals against Cationic and Anionic Dyes in Several Experimental Conditions. Colorants. 2023; 2(1):111-134. https://doi.org/10.3390/colorants2010008
Chicago/Turabian StyleNobre, Francisco, Jairo Trindade, Marcus do Nascimento, Giancarlo Souza, Otoniel Mendes, Anderson Albuquerque, Júlio Sambrano, Paulo Couceiro, Walter Brito, Yurimiler Leyet Ruiz, and et al. 2023. "Photocatalytic Properties of PbMoO4 Nanocrystals against Cationic and Anionic Dyes in Several Experimental Conditions" Colorants 2, no. 1: 111-134. https://doi.org/10.3390/colorants2010008