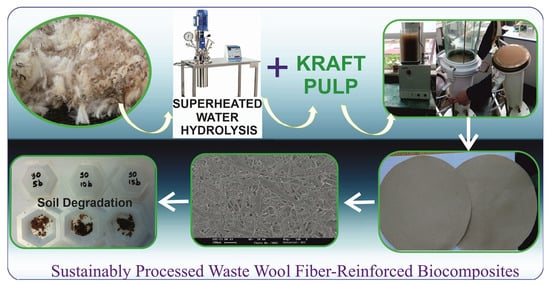
Sustainably Processed Waste Wool Fiber-Reinforced Biocomposites for Agriculture and Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
2.2.1. Superheated Water Hydrolysis of Wool
2.2.2. Biocomposite/Paper Preparation
2.3. Characterization
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.3.3. Tensile Strength
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. Thermogravimetric Analysis (TGA)
2.3.6. Biodegradation Testing in Soil
- W = Weight loss of the biocomposite after X days (%).
- Mi = Initial mass of the biocomposite (g).
- Md = Final mass of the bio composite after X days of degradation (g).
- * X = 30, 60, 90 days.
3. Result and Discussion
3.1. Scanning Electron Microscopy (SEM)
3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
3.3. Tensile Strength
3.4. Differential Scanning Calorimetry (DSC)
3.5. Thermogravimetric Analysis (TGA)
3.6. Biodegradation Testing in Soil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Confederation of European Paper Industries (CEPI). Report on Key Statistics 2019 European Pulp and Paper Industries. Available online: https://www.cepi.org/statistics/ (accessed on 31 August 2021).
- Packaging Waste Statistics—Eurostat Explained Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Packaging_waste_statistics (accessed on 31 August 2021).
- Paper & Paperboard Production & Consumption for Europe. Available online: https://paperonweb.com/Europe.htm (accessed on 31 August 2021).
- Food and Agricultural Organization (FAO). Yearbook of Forest Products 2010–2014 European Commision; Food and Agricultural Organization (FAO): Rome, Italy, 2017; p. 186. ISBN 978-92-5-131717-4. [Google Scholar]
- El-Sakhawy, M.; Lonnberg, B.; Fahmy, Y.; Ibrahim, A. Organosolv pulping. 3. Ethanol pulping of wheat straw. Cellul. Chem. Technol. 1996, 30, 161–174. [Google Scholar]
- Internal Market, Industry, Entrepreneurship and SMEs Pulp and Paper Industry European Commission. Available online: https://ec.europa.eu/growth/sectors/raw-materials/industries/forest-based/pulp-paper_en (accessed on 31 August 2021).
- Chandra, R.; Rustgi, R. Biodegradable polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Manda, B.M.K.; Blok, K.; Patel, M.K. Innovations in papermaking: An LCA of printing and writing paper from conventional and high yield pulp. Sci. Total Environ. 2012, 439, 307–320. [Google Scholar] [CrossRef]
- Marrakchi, Z.; Khiari, R.; Oueslati, H.; Mauret, E.; Mhenni, F. Pulping and papermaking properties of Tunisian Alfa stems (Stipa tenacissima)—Effects of refining process. Ind. Crops Prod. 2011, 34, 1572–1582. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Zwawi, M.; Taqi Mehran, M.; Kanthasamy, R.; Bahadar, A. Jute Based Bio and Hybrid Composites and Their Applications. Fibers 2019, 7, 77. [Google Scholar] [CrossRef] [Green Version]
- Statista Global Consumption of Paper and Cardboard 2007 to 2018. Available online: https://www.statista.com/statistics/270319/consumption-of-paper-and-cardboard-since-2006/ (accessed on 31 August 2021).
- wbcs Forest Solutions Facts & Trends: Fresh & Recycled Fiber Complementarity Technical Content by ncasi. Available online: https://www.wbcsd.org/Sector-Projects/Forest-Solutions-Group/Resources/Facts-Trends-Fresh-Recycled-Fiber-Complementarity (accessed on 31 August 2021).
- Fang, G.; Shen, K. Wheat Straw Pulping for Paper and Paperboard Production. In Global Wheat Production; IntechOpen: London, UK, 2018. [Google Scholar]
- Jha, P.; Sinha, A. Application of Rice-Straw as Raw Material for Production of Handmade Paper. IPPTA Q. J. Indian Pulp Pap. Tech. Assoc. 2011, 23, 145–148. [Google Scholar]
- Mehdikhani, H.; Jalali Torshizi, H.; Dahmardeh Ghalehno, M. Deeper insight into the morphological features of sunflower stalk as Biorefining criteria for sustainable production. Nord. Pulp Pap. Res. J. 2019, 34, 250–263. [Google Scholar] [CrossRef]
- Rainey, T.J.; Covey, G. Pulp and paper production from sugarcane bagasse. In Sugarcane-Based Biofuels Bioproducts; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 259–280. [Google Scholar]
- Wan Daud, W.R.; Law, K.N. Oil palm fibers as papermaking material: Potentials and challenges. Bioresources 2011, 6, 901–917. [Google Scholar] [CrossRef]
- García, M.M.; López, F.; Alfaro, A.; Ariza, J.; Tapias, R. The use of Tagasaste (Chamaecytisus proliferus) from different origins for biomass and paper production. Bioresour. Technol. 2008, 99, 3451–3457. [Google Scholar] [CrossRef] [PubMed]
- Brix, H.; Ye, S.; Laws, E.A.; Sun, D.; Li, G.; Ding, X.; Yuan, H.; Zhao, G.; Wang, J.; Pei, S. Large-scale management of common reed, Phragmites australis, for paper production: A case study from the Liaohe Delta, China. Ecol. Eng. 2014, 73, 760–769. [Google Scholar] [CrossRef]
- Shatalov, A.A.; Pereira, H. Papermaking fibers from giant reed (Arundo donax L.) by advanced ecologically friendly pulping and bleaching technologies. Bioresources 2006, 1, 45–61. [Google Scholar]
- Guha, S.R.D.; Gupta, R.K.; Mathur, G.M.; Sharma, Y.K. Production of Writing and Printing Papers from Prosopis juliflora. Indian For. 1970, 96, 429–432. [Google Scholar]
- Sung, Y.J.; Kim, D.S.; Lee, J.Y.; Seo, Y.B.; Im, C.K.; Gwon, W.O.; Kim, J.D. Application of Conifer Leave Powder to the Papermaking Process as an Organic Filler. J. Korea Tech. Assoc. Pulp Pap. Ind. 2014, 46, 62–68. [Google Scholar] [CrossRef]
- Pirralho, M.; Flores, D.; Sousa, V.B.; Quilhó, T.; Knapic, S.; Pereira, H. Evaluation on paper making potential of nine Eucalyptus species based on wood anatomical features. Ind. Crops Prod. 2014, 54, 327–334. [Google Scholar] [CrossRef]
- Biermann, C.J. Preface to the Second Edition. In Handbook of Pulping and Papermaking, 2nd ed.; Academic Press: San Diego, CA, USA, 1996; p. ix. ISBN 978-0-12-097362-0. [Google Scholar]
- Ashori, A. Nonwood Fibers—A Potential Source of Raw Material in Papermaking. Polym.-Plast. Technol. Eng. 2006, 45, 1133–1136. [Google Scholar] [CrossRef]
- Mohd Aripin, A. Potential of Non-Wood Fibres for Pulp and Paper-Based Industries. Master’s Thesis, Faculty of Science, Technology and Human Development, Universiti Tun Hussein Onn Malaysia, Parit Raja, Malaysia, 2014. [Google Scholar]
- EFSA Panel on Animal Health and Welfare (AHAW). Scientific Opinion on the welfare risks related to the farming of sheep for wool, meat and milk production. EFSA J. 2014, 12, 3933. [Google Scholar] [CrossRef]
- Savio, L.; Bosia, D.; Patrucco, A.; Pennacchio, R.; Piccablotto, G.; Thiebat, F. Applications of Building Insulation Products Based on Natural Wool and Hemp Fibers. In Advances in Natural Fibre Composites, Raw Materials, Processing and Analysis; Springer: Cham, Switzerland, 2018; pp. 237–247. ISBN 978-3-319-64641-1. [Google Scholar]
- Skupin, G.; Blum, R. Method for Producing Filler-Containing Paper Using Biodegradable Polyester Fibers and/or Polyalkylene Carbonate Fibers. WO 2013079378A2, 6 June 2013. [Google Scholar]
- Serrano-Ruiz, H.; Martin-Closas, L.; Pelacho, A.M. Biodegradable plastic mulches: Impact on the agricultural biotic environment. Sci. Total Environ. 2021, 750, 141228. [Google Scholar] [CrossRef]
- Espí, E.; Salmerón, A.; Fontecha, A.; García, Y.; Real, A.I. PLastic Films for Agricultural Applications. J. Plast. Film Sheeting 2006, 22, 85–102. [Google Scholar] [CrossRef]
- Le Moine, B.; Ferry, X. Plasticulture: Economy of resources. Acta Hortic. 2019, 1252, 121–130. [Google Scholar] [CrossRef]
- Briassoulis, D.; Giannoulis, A. Evaluation of the functionality of bio-based plastic mulching films. Polym. Test. 2018, 67, 99–109. [Google Scholar] [CrossRef]
- Bhavsar, P.; Patrucco, A.; Montarsolo, A.; Mossotti, R.; Rovero, G.; Giansetti, M.; Tonin, C. Superheated Water Hydrolysis of Waste Wool in a Semi-Industrial Reactor to Obtain Nitrogen Fertilizers. ACS Sustain. Chem. Eng. 2016, 4, 6722–6731. [Google Scholar] [CrossRef]
- ISO 5264-3:1979: Pulps-Laboratory Beating- Part 3: Jokro Mill Method; International Organization of Standardization: Geneva, Switzerland, 1979.
- ISO 5269-2:2004: Pulps—Preparation of Laboratory Sheets for Physical Testing—Part 2: Rapid-Köthen Method; International Organization of Standardization: Geneva, Switzerland, 2000.
- Shogren, R.L. Preparation and characterization of a biodegradable mulch: Paper coated with polymerized vegetable oils. J. Appl. Polym. Sci. 1999, 73, 2159–2167. [Google Scholar] [CrossRef]
- Ning, R.; Liang, J.; Sun, Z.; Liu, X.; Sun, W. Preparation and characterization of black biodegradable mulch films from multiple biomass materials. Polym. Degrad. Stab. 2021, 183, 109411. [Google Scholar] [CrossRef]
- Arshad, K. Biodegradation of Textile Materials. Master’s Dissertation, University of Borås, Borås, Sweden, 2011. [Google Scholar]
- Tomšič, B.; Simončič, B.; Orel, B.; Vilčnik, A.; Spreizer, H. Biodegradability of cellulose fabric modified by imidazolidinone. Carbohydr. Polym. 2007, 69, 478–488. [Google Scholar] [CrossRef]
- Schweger, B.F.; Kerr, N. Textiles collected during the temporary exhumation of a crew member from the Third Franklin Expedition: Findings and analysis. J. Int. Inst. Conserv. Can. Group 1987, 12, 9–19. [Google Scholar]
- Chen, R. A Study of Cotton Fibers Recovered from a Marine Environment. Ph.D. Dissertation, The Ohio State University, Columbus, OH, USA, 1998. [Google Scholar]
- Chen, R.; Jakes, K.A. Cellulolytic Biodegradation of Cotton Fibers from a Deep-Ocean Environment. J. Am. Inst. Conserv. 2001, 40, 91–103. [Google Scholar] [CrossRef]
- Broda, J.; Przybyło, S.; Kobiela-Mendrek, K.; Biniaś, D.; Rom, M.; Grzybowska-Pietras, J.; Laszczak, R. Biodegradation of sheep wool geotextiles. Int. Biodeterior. Biodegrad. 2016, 115, 31–38. [Google Scholar] [CrossRef]
- Jewell, P.A.; Dimbleby, G.W. The Experimental Earthwork on Overton Down, Wiltshire, England: The First Four Years. Proc. Prehist. Soc. 1966, 32, 313–342. [Google Scholar] [CrossRef]
- Peacock, E.E. Biodegradation and characterization of water-degraded archaeological textiles created for conservation research. Int. Biodeterior. Biodegrad. 1996, 38, 49–59. [Google Scholar] [CrossRef]
- Hearle, J.W.S.; Cooke, B.; Lomas, W.D. Atlas of Fibre Fracture and Damage of Textiles; Woodhead Publishing Series in Textiles; Woodhead Publishing: Manchester, UK, 1998; ISBN 9781845691271. [Google Scholar]
- Hearle, J.W.S. Fracture of common textile fibres. In Fiber Fracture; Elices, M., Llorca, J., Eds.; Elsevier Science Ltd.: Oxford, UK, 2002; pp. 329–353. ISBN 978-0-08-044104-7. [Google Scholar]
- Huson, M.G. Properties of Wool. In Handbook of Properties of Textile and Technical Fibres, 2nd ed.; The Textile Institute; Bunsell, A.R., Ed.; Elsevier: Oxford, UK, 2018; pp. 59–103. ISBN 9780081018866. [Google Scholar]
- Wood, T.M.; Phillips, D.R. Another Source of Cellulase. Nature 1969, 222, 986–987. [Google Scholar] [CrossRef]
- Béguin, P.; Aubert, J.P. The biological degradation of cellulose. FEMS Microbiol. Rev. 1994, 13, 25–58. [Google Scholar] [CrossRef]
- Wood, T.M.; McCrae, S.I. Synergism between Enzymes Involved in the Solubilization of Native Cellulose. In Hydrolysis of Cellulose: Mechanisms of Enzymatic and Acid Catalysis; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1979; Volume 181, pp. 10–181. ISBN 9780841204607. [Google Scholar]
- Marchisio, V.; Kushwaha, R.; Guarro, J. Keratinophilic fungi: Their role in nature and degradation of keratinic substrates. Biol. Derm. Other Keratinophilic Fungi 2000, 17, 86–92. [Google Scholar]
- Korniłłowicz-Kowalska, T.; Bohacz, J. Biodegradation of keratin waste: Theory and practical aspects. Waste Manag. 2011, 31, 1689–1701. [Google Scholar] [CrossRef]
- DeGaetano, D.H.; Kempton, J.B.; Rowe, W.F. Fungal tunneling of hair from a buried body. J. Forensic. Sci. 1992, 37, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Zoccola, M.; Aluigi, A.; Tonin, C. Characterisation of keratin biomass from butchery and wool industry wastes. J. Mol. Struct. 2009, 938, 35–40. [Google Scholar] [CrossRef]
- Tsobkallo, K.; Aksakal, B.; Darvish, D. Analysis of the contribution of the microfibrils and matrix to the deformation processes in wool fibers. J. Appl. Polym. Sci. 2012, 125, E168–E179. [Google Scholar] [CrossRef]
- Tang, H. Spectroscopy Analysis; Beijing University Publishing House: Beijing, China, 1992. [Google Scholar]
- Sain, M.; Panthapulakkal, S. Bioprocess preparation of wheat straw fibers and their characterization. Ind. Crops Prod. 2006, 23, 1–8. [Google Scholar] [CrossRef]
- Popescu, C.M.; Popescu, M.C.; Singurel, G.; Vasile, C.; Argyropoulos, D.S.; Willfor, S. Spectral Characterization of Eucalyptus Wood. Appl. Spectrosc. 2007, 61, 1168–1177. [Google Scholar] [CrossRef]
- Carrillo, I.; Mendonça, R.T.; Ago, M.; Rojas, O.J. Comparative study of cellulosic components isolated from different Eucalyptus species. Cellulose 2018, 25, 1011–1029. [Google Scholar] [CrossRef]
- Tsuboi, M. Infrared spectrum and crystal structure of cellulose. J. Polym. Sci. 1957, 25, 159–171. [Google Scholar] [CrossRef]
- Jonoobi, M. Chemical composition, crystallinity, and thermal degradation of bleached and unbleached kenaf bast (Hibiscus cannabinus) pulp and nanofibers. Bioresources 2009, 4, 626–639. [Google Scholar]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, L.; Liu, D.H. Peracetic acid pretreatment of sugarcane bagasse for enzymatic hydrolysis: A continued work. J. Chem. Technol. Biotechnol. 2008, 83, 950–956. [Google Scholar] [CrossRef]
- Li, R.; Fei, J.; Cai, Y.; Li, Y.; Feng, J.; Yao, J. Cellulose whiskers extracted from mulberry: A novel biomass production. Carbohydr. Polym. 2009, 76, 94–99. [Google Scholar] [CrossRef]
- Li, L.; Frey, M.; Browning, K.J. Biodegradability Study on Cotton and Polyester Fabrics. J. Eng. Fiber. Fabr. 2010, 5, 155892501000500400. [Google Scholar] [CrossRef]
- Spence, E.E. Encyclopedia of Polymer Science and Technology, 2nd ed.; Wily-Interscience: New York, NY, USA, 1987; Volume 10. [Google Scholar]
- Levy, I.; Nussinovitch, A.; Shpigel, E.; Shoseyov, O. Recombinant cellulose crosslinking protein: A novel paper-modification biomaterial. Cellulose 2002, 9, 91–98. [Google Scholar] [CrossRef]
- Xu, G.G.; Yang, C.Q.X. Comparison of the kraft paper crosslinked by polymeric carboxylic acids of large and small molecular sizes: Dry and wet performance. J. Appl. Polym. Sci. 1999, 74, 907–912. [Google Scholar] [CrossRef]
- Vineis, C.; Aluigi, A.; Tonin, C. Outstanding traits and thermal behaviour for the identification of speciality animal fibres. Text. Res. J. 2010, 81, 264–272. [Google Scholar] [CrossRef]
- Fortunati, E.; Aluigi, A.; Armentano, I.; Morena, F.; Emiliani, C.; Martino, S.; Santulli, C.; Torre, L.; Kenny, J.M.; Puglia, D. Keratins extracted from Merino wool and Brown Alpaca fibres: Thermal, mechanical and biological properties of PLLA based biocomposites. Mater. Sci. Eng. C 2015, 47, 394–406. [Google Scholar] [CrossRef]
- Eslahi, N.; Dadashian, F.; Nejad, N.H. Optimization of enzymatic hydrolysis of wool fibers for nanoparticles production using response surface methodology. Adv. Powder Technol. 2013, 24, 416–426. [Google Scholar] [CrossRef]
- Dalla Fontana, G.; Varesano, A.; Vineis, C. Effect of the Bleaching on Physical and Mechanical Properties of Different Fabrics. Fibers Polym. 2018, 19, 2590–2596. [Google Scholar] [CrossRef]
- Ibrahim, S.; El-Amoudy, E.; Shady, K.E. Thermal Analysis and Characterization of Some Cellulosic Fabrics Dyed by a New Natural Dye and Mordanted with Different Mordants. Int. J. Chem. 2011, 3, 40–54. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Bradbury, J.H.; Chapman, G.V.; King, N.L.R. The Chemical Composition of Wool II. Analysis of the Major Histological Components Produced by Ultrasonic Disintegration. Aust. J. Biol. Sci. 1965, 18, 353–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Luo, J.; Ni, A.; Bi, Y.; Yu, W. Study on Biodegradability of Wool and PLA Fibers in Natural Soil and Aqueous Medium. Adv. Mater. Res. 2013, 641–642, 82–86. [Google Scholar] [CrossRef]
- Guo, W.; Tao, J.; Yang, C.; Zhao, Q.; Song, C.; Wang, S. The rapid evaluation of material biodegradability using an improved ISO 14852 method with a microbial community. Polym. Test. 2010, 29, 832–839. [Google Scholar] [CrossRef]









| No. | Biocomposite Composition | Weight Loss % | ||
|---|---|---|---|---|
| 30 days | 60 days | 90 days | ||
| Control | (100% kraft pulp) | 12.85 | 64.30 | (~100) |
| 1 | (160 °C 90:10) | 14.60 | 67.86 | 93.90 |
| 2 | (160 °C 80:20) | 20.40 | 83.49 | 93.47 |
| 3 | (160 °C 70:30) | 34.25 | 72.25 | 89.89 |
| 4 | (160 °C 60:40) | 41.14 | 67.34 | 97.29 |
| 5 | (160 °C 50:50) | 31.52 | 78.27 | 97.75 |
| 6 | (150 °C 90:10) | 25.96 | 85.61 | (~100) |
| 7 | (150 °C 80:20) | 44.03 | 93.94 | (~100) |
| 8 | (150 °C 70:30) | 40.05 | 78.89 | 99.17 |
| 9 | (150 °C 60:40) | 46.50 | (~100) | 97.44 |
| 10 | (150 °C 50:50) | 40.71 | 93.97 | 96.58 |
| 11 | (140 °C 90:10) | 32.62 | 83.53 | 96.91 |
| 12 | (140 °C 80:20) | 27.06 | 74.60 | 91.03 |
| 13 | (140 °C 70:30) | 30.29 | 58.41 | 96.86 |
| 14 | (140 °C 60:40) | 42.92 | 71.41 | 95.43 |
| 15 | (140 °C 50:50) | 42.32 | 71.80 | 98.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhavsar, P.; Balan, T.; Dalla Fontana, G.; Zoccola, M.; Patrucco, A.; Tonin, C. Sustainably Processed Waste Wool Fiber-Reinforced Biocomposites for Agriculture and Packaging Applications. Fibers 2021, 9, 55. https://doi.org/10.3390/fib9090055
Bhavsar P, Balan T, Dalla Fontana G, Zoccola M, Patrucco A, Tonin C. Sustainably Processed Waste Wool Fiber-Reinforced Biocomposites for Agriculture and Packaging Applications. Fibers. 2021; 9(9):55. https://doi.org/10.3390/fib9090055
Chicago/Turabian StyleBhavsar, Parag, Tudor Balan, Giulia Dalla Fontana, Marina Zoccola, Alessia Patrucco, and Claudio Tonin. 2021. "Sustainably Processed Waste Wool Fiber-Reinforced Biocomposites for Agriculture and Packaging Applications" Fibers 9, no. 9: 55. https://doi.org/10.3390/fib9090055