Development of Thin Films from Thermomechanical Pulp Nanofibers of Radiata Pine (Pinus radiata D. Don) for Applications in Bio-Based Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
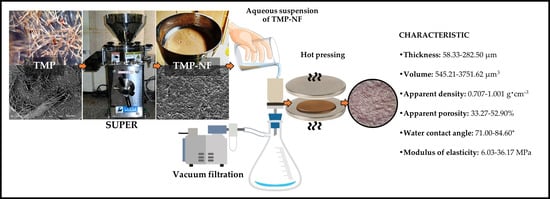
Obtaining Nanofibers from Mechanical Pulp and Film Development
2.2. Methodology
2.2.1. Obtaining Nanofibers from Thermomechanical Pulp
2.2.2. Development of CNF and TMP-NF Films
2.3. Characterization Methods
2.3.1. Determination of the Constituents and Morphological Characterization of the Thermomechanical Pulp
2.3.2. Determination of the Yield of TMP-NF and Morphological Characterization of TMP-NF by Scanning Electron Microscopy (SEM) and Atomic Force Microscopy (AFM)
2.3.3. X-ray Diffraction Analysis (XRD) of CNF and TMP-NF
2.3.4. Characterization of CNF and TMP-NF by Fourier-Transform Infrared Spectroscopy with Attenuated Total Reflection (FTIR-ATR)
2.3.5. Morphological, Physical, Structural, and Surface Properties of the Developed Films
Morphology
Determination of the Thickness, Volume, Apparent Density, and Apparent Porosity of the Developed Films
Water Contact Angle
Mechanical Analysis of Developed Films
2.4. Statistical Analysis
3. Results and Discussion
3.1. Determination of the Constituents of the Thermomechanical Pulp, Chemical Characterization
3.1.1. Morphological Characterization of Thermomechanical Pulp Using SEM
3.1.2. Process for Obtaining TMP-NF Using Micro-Grinding
3.1.3. Determination of the Yield of TMP-NF and Morphological Characterization Using SEM and AFM to TMP-NF after the Size Reduction Process
3.1.4. Crystallographic Analysis Using XRD of CNF and TMP-NF
3.1.5. Analysis Using FTIR-ATR for CNF and TMP-NF
3.2. Characterization of the Developed Films: Morphological, Physical, Structural, and Surface Properties
3.2.1. Surface Morphology of Developed Films
3.2.2. Thickness and Volume of the Developed Films
3.2.3. Structural Properties of the Developed Films, Apparent Density, and Apparent Porosity
3.2.4. Water Contact Angle
3.3. Mechanical Analysis of Developed Films
Modulus of Elasticity, Tensile Strength, and Elongation at Failure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohanty, A.K.; Misra, M.; Hinrichsen, G. Biofibres, Biodegradable Polymers and Biocomposites: An Overview. Macromol. Mater. Eng. 2000, 276–277, 1–24. [Google Scholar] [CrossRef]
- Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Emerging Technologies for the Production of Nanocellulose from Lignocellulosic Biomass. Carbohydr. Polym. 2022, 285, 119258. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Chae, M.; Bressler, D.C. Composite Materials with Bast Fibres: Structural, Technical, and Environmental Properties. Prog. Mater. Sci. 2016, 83, 1–23. [Google Scholar] [CrossRef]
- Tyagi, P.; Gutierrez, J.N.; Nathani, V.; Lucia, L.A.; Rojas, O.J.; Hubbe, M.A.; Pal, L. Hydrothermal and Mechanically Generated Hemp Hurd Nanofibers for Sustainable Barrier Coatings/Films. Ind. Crops Prod. 2021, 168, 113582. [Google Scholar] [CrossRef]
- Wang, X.; Xia, Q.; Jing, S.; Li, C.; Chen, Q.; Chen, B.; Pang, Z.; Jiang, B.; Gan, W.; Chen, G.; et al. Strong, Hydrostable, and Degradable Straws Based on Cellulose-Lignin Reinforced Composites. Small 2021, 17, 2008011. [Google Scholar] [CrossRef]
- Bian, H.; Dong, M.; Chen, L.; Zhou, X.; Wang, R.; Jiao, L.; Ji, X.; Dai, H. On-Demand Regulation of Lignocellulosic Nanofibrils Based on Rapid Fractionation Using Acid Hydrotrope: Kinetic Study and Characterization. ACS Sustain. Chem. Eng. 2020, 8, 9569–9577. [Google Scholar] [CrossRef]
- Diop, C.I.K.; Tajvidi, M.; Bilodeau, M.A.; Bousfield, D.W.; Hunt, J.F. Isolation of Lignocellulose Nanofibrils (LCNF) and Application as Adhesive Replacement in Wood Composites: Example of Fiberboard. Cellulose 2017, 24, 3037–3050. [Google Scholar] [CrossRef]
- Osong, S.H.; Norgren, S.; Engstrand, P. An Approach to Produce Nano-Ligno-Cellulose from Mechanical Pulp Fine Materials. Nord. Pulp Pap. Res. J. 2013, 28, 472–479. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Wu, T.; Ma, J.; Lu, H.; Zhou, X. A Systematic Study of Lignocellulose Nanofibrils (LCNF) Prepared from Wheat Straw by Varied Acid Pretreatments. Ind. Crops Prod. 2022, 185, 115126. [Google Scholar] [CrossRef]
- Taniguchi, T.; Okamura, K. New Films Produced from Microfibrillated Natural Fibres. Polym. Int. 1998, 47, 291–294. [Google Scholar] [CrossRef]
- Vergara-Figueroa, J.; Alejandro-Martín, S.; Pesenti, H.; Cerda, F.; Fernández-Pérez, A.; Gacitúa, W. Obtaining Nanoparticles of Chilean Natural Zeolite and Its Ion Exchange with Copper Salt (Cu2+) for Antibacterial Applications. Materials 2019, 12, 2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergara-Figueroa, J.; Alejandro-Martin, S.; Cerda-Leal, F.; Gacitúa, W. Dual Electrospinning of a Nanocomposites Biofilm: Potential Use as an Antimicrobial Barrier. Mater. Today Commun. 2020, 25, 12. [Google Scholar] [CrossRef]
- Horseman, T.; Tajvidi, M.; Diop, C.I.K.; Gardner, D.J. Preparation and Property Assessment of Neat Lignocellulose Nanofibrils (LCNF) and Their Composite Films. Cellulose 2017, 24, 2455–2468. [Google Scholar] [CrossRef]
- Ko, F.; Wan, Y. Introduction to Nanofiber Materials, 1st ed.; Cambridge University Press: New York, NY, USA, USA, 2014; Volume 9780521879, ISBN 978-0-521-87983-5. [Google Scholar]
- Hu, C.; Yang, Y.; Lin, Y.; Wang, L.; Ma, R.; Zhang, Y.; Feng, X.; Wu, J.; Chen, L.; Shao, L. GO-Based Antibacterial Composites: Application and Design Strategies. Adv. Drug Deliv. Rev. 2021, 178, 113967. [Google Scholar] [CrossRef] [PubMed]
- Amini, E.; Hafez, I.; Tajvidi, M.; Bousfield, D.W. Cellulose and Lignocellulose Nanofibril Suspensions and Films: A Comparison. Carbohydr. Polym. 2020, 250, 117011. [Google Scholar] [CrossRef]
- Farooq, M.; Zou, T.; Riviere, G.; Sipponen, M.H.; Österberg, M. Strong, Ductile, and Waterproof Cellulose Nanofibril Composite Films with Colloidal Lignin Particles. Biomacromolecules 2019, 20, 693–704. [Google Scholar] [CrossRef]
- Kontturi, K.S.; Lee, K.Y.; Jones, M.P.; Sampson, W.W.; Bismarck, A.; Kontturi, E. Influence of Biological Origin on the Tensile Properties of Cellulose Nanopapers. Cellulose 2021, 28, 6619–6628. [Google Scholar] [CrossRef]
- Österberg, M.; Vartiainen, J.; Lucenius, J.; Hippi, U.; Seppälä, J.; Serimaa, R.; Laine, J. A Fast Method to Produce Strong NFC Films as a Platform for Barrier and Functional Materials. ACS Appl. Mater. Interfaces 2013, 5, 4640–4647. [Google Scholar] [CrossRef]
- Parameswaran, C.; Gupta, D. Large Area Flexible Pressure/Strain Sensors and Arrays Using Nanomaterials and Printing Techniques. Nano Converg. 2019, 6, 28. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Li, X.; Jo, H.; Sideeq Bhat, K.; Kim, S.; An, J.; Kang, J.W.; Lim, S. Mulberry Paper-Based Graphene Strain Sensor for Wearable Electronics with High Mechanical Strength. Sens. Actuators A Phys. 2020, 301, 111697. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-Derived Materials for Green Electronics, Biological Devices, and Energy Applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Park, S.Y.; Yook, S.; Shin, H.; Lee, H.L.; Youn, H.J. A Waterproof Cellulose Nanofibril Sheet Prepared by the Deposition of an Alkyl Ketene Dimer on a Controlled Porous Structure. Cellulose 2022, 29, 6645–6657. [Google Scholar] [CrossRef] [PubMed]
- Junka, K.; Filpponen, I.; Johansson, L.S.; Kontturi, E.; Rojas, O.J.; Laine, J. A Method for the Heterogeneous Modification of Nanofibrillar Cellulose in Aqueous Media. Carbohydr. Polym. 2012, 100, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Kalia, S.; Boufi, S.; Celli, A.; Kango, S. Nanofibrillated Cellulose: Surface Modification and Potential Applications. Colloid Polym. Sci. 2014, 292, 5–31. [Google Scholar] [CrossRef]
- MASUKO SANGYIO CO. LTD. Ultra-Fine Friction Grinder “Supermasscolloider”. Available online: http://www.masuko.com/English/product/Masscolloder.html (accessed on 10 January 2016).
- Lu, H.; Zhang, L.; Yan, M.; Wang, K.; Jiang, J. Screw Extrusion Pretreatment for High-Yield Lignocellulose Nanofibrils (LCNF) Production from Wood Biomass and Non-Wood Biomass. Carbohydr. Polym. 2022, 277, 118897. [Google Scholar] [CrossRef]
- TAPPI TAPPI Standards, Technical Information Papers, and Useful Methods Online. Available online: https://tappi.micronexx.com/ (accessed on 1 November 2021).
- TAPPI. Acid-Insoluble Lignin in Wood and Pulp (Reaffirmation of T 222 Om-02); TAPPI: Peachtree Corners, GA, USA, 2006; pp. 1–7. [Google Scholar]
- TAPPI. T211 Ash in Wood, Pulp, Paper and Paperboard: Combustion at 525 °C; TAPPI: Peachtree Corners, GA, USA, 2007; Volume T211 om-02, p. 7. [Google Scholar]
- TAPPI. Sampling and Preparing Wood for Analysis (Proposed Revision of T 257 Cm-02 as a Standard Practice); TAPPI: Peachtree Corners, GA, USA, 2012. [Google Scholar]
- Yan, M.; Zhang, L.; Ma, J.; Lu, H.; Zhou, X. Stable Suspensions of Lignocellulose Nanofibrils (LCNFs) Dispersed in Organic Solvents. ACS Sustain. Chem. Eng. 2020, 8, 15989–15997. [Google Scholar] [CrossRef]
- Ju, X.; Bowden, M.; Brown, E.E.; Zhang, X. An Improved X-ray Diffraction Method for Cellulose Crystallinity Measurement. Carbohydr. Polym. 2015, 123, 476–481. [Google Scholar] [CrossRef] [Green Version]
- Coelho, A. TOPAS-Academic V7. Available online: http://www.topas-academic.net/ (accessed on 20 September 2022).
- Young, R.A. The Rietveld Method, 5th ed.; Oxford University Press: New York, NY, USA, 1993; Volume 5, ISBN 0198555776. [Google Scholar]
- Dinnebier, R.; Leineweber, A.; Evans, J. 5. Quantitative Phase Analysis. In Rietveld Refinement; De Gruyter: Berlin/Boston, Germany, 2019; pp. 131–150. ISBN 9783110461381. [Google Scholar]
- March, A. Mathematische Theorie Der Regelung Nach Der Korngestah Bei Affiner Deformation. Z. Krist.-Cryst. Mater. 1932, 81, 285–297. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Sim, K.; Youn, H.J. Preparation of Porous Sheets with High Mechanical Strength by the Addition of Cellulose Nanofibrils. Cellulose 2016, 23, 1383–1392. [Google Scholar] [CrossRef]
- Reyes-Rodriguez, J.L.; Escorihuela, J.; Garcia-Bernabe, A.; Gimenez, E.; Solorza-Feria, O.; Compan, V. Proton Conducting Electrospun Sulfonated Polyether Ether Ketone Graphene Oxide Composite Membranes. RSC Adv. 2017, 7, 53481–53491. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.C.; Tze, W.T.Y.; Chang, F.C. Effects of Nanocellulose Formulation on Physicomechanical Properties of Aquazol–Nanocellulose Composites. Cellulose 2020, 27, 5757–5769. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Cybulska, J.; Kozioł, A.; Pieczywek, P.M.; Zdunek, A. Simultaneous Influence of Pectin and Xyloglucan on Structure and Mechanical Properties of Bacterial Cellulose Composites. Carbohydr. Polym. 2017, 174, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Andersson, R.L.; Salajkova, M.; Mallon, P.E.; Berglund, L.A.; Hedenqvist, M.S.; Olsson, R.T. Micromechanical Tensile Testing of Cellulose-Reinforced Electrospun Fibers Using a Template Transfer Method (TTM). J. Polym. Environ. 2012, 20, 967–975. [Google Scholar] [CrossRef]
- Berrocal, A.; Baeza, J.; Rodríguez, J.; Espinosa, M.; Freer, J. Effect of Tree Age on Variation of Pinus Radiata D. Don Chemical Composition. J. Chil. Chem. Soc. 2004, 49, 251–256. [Google Scholar] [CrossRef]
- Row, R.M. (Ed.) Handbook of Wood Chemistry and Wood Composites, 1st ed.; Taylor & Francis: Boca Raton, FL, USA, 2005; Volume 43, ISBN 0849315883. [Google Scholar]
- Rowell, R.M. Handbook of Wood Chemistry and Wood Composites, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 75–98. ISBN 9781439853818. [Google Scholar]
- Nair, S.S.; Kuo, P.Y.; Chen, H.; Yan, N. Investigating the Effect of Lignin on the Mechanical, Thermal, and Barrier Properties of Cellulose Nanofibril Reinforced Epoxy Composite. Ind. Crops Prod. 2017, 100, 208–217. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, H.; Yu, J.; Fan, Y.; Ma, J.; Wang, Z. Contribution of Lignin to the Microstructure and Physical Performance of Three-Dimensional Lignocellulose Hydrogels. Cellulose 2019, 26, 2375–2388. [Google Scholar] [CrossRef]
- Cusola, O.; Rojas, O.J.; Roncero, M.B. Lignin Particles for Multifunctional Membranes, Antioxidative Microfiltration, Patterning, and 3D Structuring. ACS Appl. Mater. Interfaces 2019, 11, 45226–45236. [Google Scholar] [CrossRef]
- O’sullvian, A.C. Cellulose: The Structure Slowly Unravels. Cellulose 1997, 4, 173–207. [Google Scholar] [CrossRef]
- Hosemann, R.; Hindeleh, A.M. Structure of Crystalline and Paracrystalline Condensed Matter. J. Macromol. Sci. Part B 1995, 34, 327–356. [Google Scholar] [CrossRef]
- Paul Mclean, J.; Jin, G.; Brennan, M.; Nieuwoudt, M.K.; Harris, P.J. Using NIR and ATR-FTIR Spectroscopy to Rapidly Detect Compression Wood in Pinus Radiata. Can. J. For. Res. 2014, 44, 820–830. [Google Scholar] [CrossRef]
- Yang, Y.P.; Zhang, Y.; Lang, Y.X.; Yu, M.H. Structural ATR-IR Analysis of Cellulose Fibers Prepared from a NaOH Complex Aqueous Solution. IOP Conf. Ser. Mater. Sci. Eng. 2017, 213, 8. [Google Scholar] [CrossRef] [Green Version]
- Castillo, R.d.P.; Araya, J.; Troncoso, E.; Vinet, S.; Freer, J. Fourier Transform Infrared Imaging and Microscopy Studies of Pinus Radiata Pulps Regarding the Simultaneous Saccharification and Fermentation Process. Anal. Chim. Acta 2015, 866, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Robles, J.; Sánchez, R.; Espinosa, E.; Savy, D.; Mazzei, P.; Piccolo, A.; Rodríguez, A. Isolation and Characterization of Gramineae and Fabaceae Soda Lignins. Int. J. Mol. Sci. 2017, 18, 327. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Robles, J.; Soledad, M.; Tamminen, T.; Rodríguez, A.; Larrañeta, E.; Jääskeläinen, A. Lignin-Based Hydrogels with “ Super-Swelling ” Capacities for Dye Removal. Int. J. Biol. Macromol. 2018, 115, 1249–1259. [Google Scholar] [CrossRef] [Green Version]
- Rojo, E.; Peresin, M.S.; Sampson, W.W.; Hoeger, I.C.; Vartiainen, J.; Laine, J.; Rojas, O.J. Comprehensive Elucidation of the Effect of Residual Lignin on the Physical, Barrier, Mechanical and Surface Properties of Nanocellulose Films. Green Chem. 2015, 17, 1853–1866. [Google Scholar] [CrossRef] [Green Version]
- Gibson, L.J. The Hierarchical Structure and Mechanics of Plant Materials. J. R. Soc. Interface 2012, 9, 2749–2766. [Google Scholar] [CrossRef]














| Factors | Levels (%) | Response Variable |
|---|---|---|
| CNF TMP-NF | 0.10 0.25 0.50 1.00 | Thickness (µm) Volume (µm3) Apparent density (g∙cm−3) Apparent porosity (%) Water contact angle (°) Modulus of elasticity (MPa) Tensile strength (MPa) Elongation at failure (%) |
| Sample | CNF | TMP-NF | |||||
|---|---|---|---|---|---|---|---|
| Proportion Phases (%) | Alpha | Beta | Amorphous | Alpha | Beta | Amorphous | |
| 18.5 | 80.5 | 1.0 | 9.2 | 62.5 | 28.3 | ||
| Average crystalline Domain Size (nm) | 6.8 | 6.1 | 7.5 | 8.4 | |||
| Lattice Parameters | Lengths | a: 6.100 Å | a: 7.996 Å | a: 6.089 Å | a: 7.980 Å | ||
| b: 5.994 Å | b: 8.478 Å | b: 5.833 Å | b: 8.162 Å | ||||
| c: 11.298 Å | c: 10.275 Å | c: 10.528 Å | c: 10.426 Å | ||||
| Angles | α: 118.020° | α: 117.592° | |||||
| β: 110.176° | β: 112.113° | ||||||
| γ: 82.005° | γ: 95.440° | γ: 82.064° | γ: 96.890° | ||||
| Segal Crystalline index (%) | 66 | 43 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergara-Figueroa, J.; Erazo, O.; Pesenti, H.; Valenzuela, P.; Fernández-Pérez, A.; Gacitúa, W. Development of Thin Films from Thermomechanical Pulp Nanofibers of Radiata Pine (Pinus radiata D. Don) for Applications in Bio-Based Nanocomposites. Fibers 2023, 11, 1. https://doi.org/10.3390/fib11010001
Vergara-Figueroa J, Erazo O, Pesenti H, Valenzuela P, Fernández-Pérez A, Gacitúa W. Development of Thin Films from Thermomechanical Pulp Nanofibers of Radiata Pine (Pinus radiata D. Don) for Applications in Bio-Based Nanocomposites. Fibers. 2023; 11(1):1. https://doi.org/10.3390/fib11010001
Chicago/Turabian StyleVergara-Figueroa, Judith, Oswaldo Erazo, Héctor Pesenti, Paulina Valenzuela, Arturo Fernández-Pérez, and William Gacitúa. 2023. "Development of Thin Films from Thermomechanical Pulp Nanofibers of Radiata Pine (Pinus radiata D. Don) for Applications in Bio-Based Nanocomposites" Fibers 11, no. 1: 1. https://doi.org/10.3390/fib11010001