Effect of Chitosan-Ascorbic Acid Coatings on the Refrigerated Storage Stability of Fresh-Cut Apples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Consumables
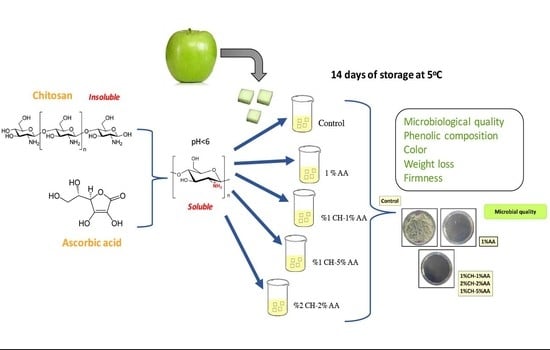
2.2. Preparation of Fresh-Cut Apples and Dipping Solutions
2.3. Treatments of Fresh-Cut Apples
2.4. Microbiological Analyses and Kinetic Modelling of Microbial Growth
2.5. Measurement of Weight Loss
2.6. Measurement of Firmness
2.7. Measurement of Color
2.8. Analysis of Phenolic Compounds
2.9. Statistical Analysis
3. Results and Discussion
3.1. Weight Loss
3.2. Firmness
3.3. Color
3.4. Phenolic Compounds
3.5. Microbialanalyses and Shelf Life Modelling
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marszałek, K.; Woźniak, Ł.; Barba, F.J.; Skąpska, S.; Lorenzo, J.M.; Zambon, A.; Spilimbergo, S. Enzymatic, physicochemical, nutritional and phytochemical profile changes of apple (Golden Delicious L.) juice under supercritical carbon dioxide and long-term cold storage. Food Chem. 2018, 268, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.; Bi, J.; Liu, X.; Li, X.; Guo, C. Polyphenols accumulation effects on surface color variation in apple slices hot air drying process. LWT 2019, 108, 421–428. [Google Scholar] [CrossRef]
- Guo, J.; Yue, T.L.; Yuan, Y.H.; Wang, Y.T. Chemometric Classification of Apple Juices According to Variety and Geographical Origin Based on Polyphenolic Profiles. J. Agric. Food Chem. 2013, 61, 6949–6963. [Google Scholar] [CrossRef] [PubMed]
- Kabelitz, T.; Hassenberg, K. Control of apple surface microflora for fresh-cut produce by post-harvest hot-water treatment. LWT 2018, 98, 492–499. [Google Scholar] [CrossRef]
- Luo, Y.; Barbosa Canovas, G.V. Enzymatic browning and its inhibition in new apple cultivars slices using 4-hexylresorcinol in combination with ascorbic acid. Food Sci. Technol. Int. 1997, 3, 195–201. [Google Scholar] [CrossRef]
- Mcevily, A.J.; Iyengar, R.; Otwell, W.S. Inhibition of Enzymatic Browning in Foods and Beverages. Crit. Rev. Food Sci. 1992, 32, 253–273. [Google Scholar] [CrossRef]
- Saba, M.K.; Sogvar, O.B. Combination of carboxymethyl cellulose-based coatings with calcium and ascorbic acid impacts in browning and quality of fresh-cut apples. LWT Food Sci. Technol. 2016, 66, 165–171. [Google Scholar] [CrossRef]
- Putnik, P.; Kovacevic, D.B.; Herceg, K.; Pavkov, I.; Zoric, Z.; Levaj, B. Effects of modified atmosphere, anti-browning treatments and ultrasound on the polyphenolic stability, antioxidant capacity and microbial growth in fresh-cut apples. J. Food Process. Eng. 2017, 40, e12359. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Aguiló-Aguayo, I.; Martín-Belloso, O. Inhibition of Browning on Fresh-cut Pear Wedges by Natural Compounds. J. Food Sci. 2006, 71, S216–S224. [Google Scholar] [CrossRef]
- Terefe, N.S.; Tepper, P.; Ullman, A.; Knoerzer, K.; Juliano, P. High pressure thermal processing of pears: Effect on endogenous enzyme activity and related quality attributes. Innov. Food Sci. Emerg. 2016, 33, 56–66. [Google Scholar] [CrossRef]
- Alves, M.M.; Goncalves, M.P.; Rocha, C.M.R. Effect of ferulic acid on the performance of soy protein isolate-based edible coatings applied to fresh-cut apples. LWT Food Sci. Technol. 2017, 80, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Sanchis, E.; Ghidelli, C.; Sheth, C.C.; Mateos, M.; Palou, L.; Perez-Gago, M.B. Integration of antimicrobial pectin-based edible coating and active modified atmosphere packaging to preserve the quality and microbial safety of fresh-cut persimmon (Diospyros kaki Thunb. cv. Rojo Brillante). J. Sci. Food Agric. 2017, 97, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Marquez, G.R.; Di Pierro, P.; Mariniello, L.; Esposito, M.; Giosafatto, C.V.L.; Porta, R. Fresh-cut fruit and vegetable coatings by transglutaminase-crosslinked whey protein/pectin edible films. LWT Food Sci. Technol. 2017, 75, 124–130. [Google Scholar] [CrossRef]
- Moreira, M.R.; Alvarez, M.V.; Martin-Belloso, O.; Soliva-Fortuny, R. Effects of pulsed light treatments and pectin edible coatings on the quality of fresh-cut apples: A hurdle technology approach. J. Sci. Food Agric. 2017, 97, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Grau, M.A.; Sobrino-Lopez, A.; Tapia, M.S.; Martin-Belloso, O. Browning inhibition in fresh-cut ‘fuji’ apple slices by natural antibrowning agents. J. Food Sci. 2006, 71, S59–S65. [Google Scholar] [CrossRef]
- Park, H.J. Development of advanced edible coatings for fruits. Trends Food Sci. Technol. 1999, 10, 254–260. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Devlieghere, F.; Vermeulen, A.; Debevere, J. Chitosan: Antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004, 21, 703–714. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Kerch, G. Chitosan films and coatings prevent losses of fresh fruit nutritional quality: A review. Trends Food Sci. Technol. 2015, 46, 159–166. [Google Scholar] [CrossRef]
- Wang, S.Y.; Gao, H. Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (Fragaria x aranassa Duch.). LWT Food Sci. Technol. 2013, 52, 71–79. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z.X. Recent developments in novel shelf life extension technologies of fresh-cut fruits and vegetables. Trends Food Sci. Technol. 2017, 64, 23–38. [Google Scholar] [CrossRef] [Green Version]
- Coma, V.; Martial-Gros, A.; Garreau, S.; Copinet, A.; Salin, F.; Deschamps, A. Edible Antimicrobial Films Based on Chitosan Matrix. J. Food Sci. 2002, 67, 1162–1169. [Google Scholar] [CrossRef]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.-J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Vásconez, M.B.; Flores, S.K.; Campos, C.A.; Alvarado, J.; Gerschenson, L.N. Antimicrobial activity and physical properties of chitosan–tapioca starch based edible films and coatings. Food Res. Int. 2009, 42, 762–769. [Google Scholar] [CrossRef]
- Ge, H.-C.; Luo, D.-K. Preparation of carboxymethyl chitosan in aqueous solution under microwave irradiation. Carbohydr. Res. 2005, 340, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Chien, P.-J.; Sheu, F.; Yang, F.-H. Effects of edible chitosan coating on quality and shelf life of sliced mango fruit. J. Food Eng. 2007, 78, 225–229. [Google Scholar] [CrossRef]
- Jongsri, P.; Wangsomboondee, T.; Rojsitthisak, P.; Seraypheap, K. Effect of molecular weights of chitosan coating on postharvest quality and physicochemical characteristics of mango fruit. LWT Food Sci. Technol. 2016, 73, 28–36. [Google Scholar] [CrossRef]
- Tezotto-Uliana, J.V.; Fargoni, G.P.; Geerdink, G.M.; Kluge, R.A. Chitosan applications pre- or postharvest prolong raspberry shelf-life quality. Postharvest Biol. Technol. 2014, 91, 72–77. [Google Scholar] [CrossRef]
- Qi, H.P.; Hu, W.Z.; Jiang, A.L.; Tian, M.X.; Li, Y.Q. Extending shelf-life of Fresh-cut ‘Fuji’ apples with chitosan-coatings. Innov. Food Sci. Emerg. 2011, 12, 62–66. [Google Scholar] [CrossRef]
- Liu, X.; Ren, J.; Zhu, Y.; Han, W.; Xuan, H.; Ge, L. The preservation effect of ascorbic acid and calcium chloride modified chitosan coating on fresh-cut apples at room temperature. Coll. Surf. A Physicochem. Eng. Asp. 2016, 502, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Ngamchuachit, P.; Sivertsen, H.K.; Mitcham, E.J.; Barrett, D.M. Effectiveness of calcium chloride and calcium lactate on maintenance of textural and sensory qualities of fresh-cut mangos. J. Food Sci. 2014, 79, C786–C794. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; van’t Riet, K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [PubMed]
- Gökmen, V.; Mogol, B.A. Computer vision-based image analysis for rapid detection of acrylamide in heated foods. Qual. Assur. Saf. Crop. Foods 2010, 2, 203–207. [Google Scholar] [CrossRef]
- Özdemir, K.S.; Yılmaz, C.; Durmaz, G.; Gökmen, V. Hazelnut skin powder: A new brown colored functional ingredient. Food Res. Int. 2014, 65, 291–297. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Q.M.; Cao, J.K.; Jiang, W.B. Effects of chitosan coating on postharvest quality of mango (Mangifera indica L. cv. Tainong) fruits. J. Food Process. Preserv. 2008, 32, 770–784. [Google Scholar] [CrossRef]
- Elghaouth, A.; Arul, J.; Ponnampalam, R.; Boulet, M. Use of Chitosan Coating to Reduce Water-Loss and Maintain Quality of Cucumber and Bell Pepper Fruits. J. Food Process. Preserv. 1991, 15, 359–368. [Google Scholar]
- Dong, H.Q.; Cheng, L.Y.; Tan, J.H.; Zheng, K.W.; Jiang, Y.M. Effects of chitosan coating on quality and shelf life of peeled litchi fruit. J. Food Eng. 2004, 64, 355–358. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Sobrino-López, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Shelf-life extension of fresh-cut “Fuji” apples at different ripeness stages using natural substances. Postharvest Biol. Technol. 2007, 45, 265–275. [Google Scholar] [CrossRef]
- Hong, K.Q.; Xie, J.H.; Zhang, L.B.; Sun, D.Q.; Gong, D.Q. Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Sci. Hortic. 2012, 144, 172–178. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Zahedi, S.M.; Abadía, J.; Karimi, M. Effects of postharvest treatments with chitosan and putrescine to maintain quality and extend shelf-life of two banana cultivars. Food Sci. Nutr. 2018, 6, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zhu, Z.; Zhang, P. Effects of chitosan–glucose complex coating on postharvest quality and shelf life of table grapes. Carbohydr. Polym. 2013, 95, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.L.; Cabral, M.F.; Germano, T.A.; de Carvalho, W.M.; Brasil, I.M.; Gallão, M.I.; Moura, C.F.H.; Lopes, M.M.A.; de Miranda, M.R.A. Chitosan coating with trans-cinnamaldehyde improves structural integrity and antioxidant metabolism of fresh-cut melon. Postharvest Biol. Technol. 2016, 113, 29–39. [Google Scholar] [CrossRef]
- Ali, H.M.; El-Gizawy, A.M.; El-Bassiouny, R.E.I.; Saleh, M.A. Browning inhibition mechanisms by cysteine, ascorbic acid and citric acid, and identifying PPO-catechol-cysteine reaction products. J. Food Sci. Technol. 2015, 52, 3651–3659. [Google Scholar] [CrossRef] [PubMed]
- Oszmianski, J.; Lee, C.Y. Enzymic oxidative reaction of catechin and chlorogenic acid in a model system. J. Agric. Food Chem. 1990, 38, 1202–1204. [Google Scholar] [CrossRef]
- Rico, D.; Martín-Diana, A.B.; Barat, J.M.; Barry-Ryan, C. Extending and measuring the quality of fresh-cut fruit and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 373–386. [Google Scholar] [CrossRef]
- Cadogan, E.I.; Lee, C.-H.; Popuri, S.R.; Lin, H.-Y. Effect of Solvent on Physico-Chemical Properties and Antibacterial Activity of Chitosan Membranes. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 708–715. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ponce, A.G.; Moreira, M.D. Antimicrobial efficiency of chitosan coating enriched with bioactive compounds to improve the safety of fresh cut broccoli. LWT Food Sci. Technol. 2013, 50, 78–87. [Google Scholar] [CrossRef]
- Karagoz, S.; Demirdoven, A. Effect of chitosan coatings with and without Stevia rebaudiana and modified atmosphere packaging on quality of cold stored fresh-cut apples. LWT Food Sci. Technol. 2019, 108, 332–337. [Google Scholar] [CrossRef]
- Pushkala, R.; Parvathy, K.R.; Srividya, N. Chitosan powder coating, a novel simple technique for enhancement of shelf life quality of carrot shreds stored in macro perforated LDPE packs. Innov. Food Sci. Emerg. Technol. 2012, 16, 11–20. [Google Scholar] [CrossRef]



| Treatment | Storage Time (day) | ||
|---|---|---|---|
| 3 | 7 | 14 | |
| Control | 0.47 ± 0.08 aA | 0.55 ± 0.23 aAB | 3.45 ± 0.40 bC |
| 1%ASC | 0.45 ± 0.01 aA | 0.31 ± 0.02 aA | 5.38 ± 0.39 bD |
| 1%CH–1%ASC | 1.04 ± 0.12 aB | 1.00 ± 0.24 aB | 1.31 ± 0.26 aA |
| 1%CH–5%ASC | 0.94 ± 0.26 aB | 0.92 ± 0.31 aB | 2.11 ± 0.72 aAB |
| 2%CH–2%ASC | 0.87 ± 0.04 aB | 1.65 ± 0.18 bC | 2.63 ± 0.33 cBC |
| Treatment | Storage Time (Day) | |||
|---|---|---|---|---|
| 1 | 3 | 7 | 14 | |
| Control | 20.2 ± 2.2 bA | 17.6 ± 0.3 aA | 18.6 ± 1.8 abA | − |
| 1%ASC | 20.9 ± 1.7 aA | 19.6 ± 2.6 aA | 20.8 ± 1.8 aA | − |
| 1%CH–1%ASC | 20.6 ± 1.2 aA | 19.0 ± 2.8 aA | 19.6 ± 2.2 aA | − |
| 1%CH–5%ASC | 19.6 ± 2.1 aA | 20.1 ± 2.3 aA | 19.3 ± 2.3 aA | 18.1 ± 3.5 aA |
| 2%CH–2%ASC | 19.1 ± 1.7 aA | 19.4 ± 1.9 aA | 19.3 ± 2.4 aA | 18.4 ± 4.6 aA |
| Analysis | Day | Control | 1%CH–1%ASC | 1%CH–5%ASC |
|---|---|---|---|---|
| Catechin | 0 | 1.00 ± 0.00 aA | 1.00 ± 0.00 aA | 1.00 ± 0.00 abA |
| 6 | 0.17 ± 0.02 bA | 0.80 ± 0.12 bB | 1.17 ± 0.09 aC | |
| 12 | 0.13 ± 0.01 bcA | 0.21 ± 0.02 cA | 0.95 ± 0.09 bB | |
| 18 | 0.10 ± 0.02 cA | 0.20 ± 0.00 cA | 0.89 ± 0.07 bB | |
| Epicatechin | 0 | 1.00 ± 0.00 aA | 1.00 ± 0.0 aA | 1.00 ± 0.00 aA |
| 6 | 0.35 ± 0.07 bcA | 0.89 ± 0.09 aB | 0.75 ± 0.11 abB | |
| 12 | 0.46 ± 0.00 bA | 0.42 ± 0.01 bA | 0.55 ± 0.09 bA | |
| 18 | 0.30 ± 0.00 cA | 0.28 ± 0.03 cA | 0.71 ± 0.19 abB | |
| Chlorogenic acid | 0 | 1.00 ± 0.00 aA | 1.00 ± 0.00 aA | 1.00 ± 0.00 aA |
| 6 | 0.93 ± 0.00 aA | 1.05 ± 0.11 aA | 1.06 ± 0.02 aA | |
| 12 | 0.99 ± 0.08 aA | 1.30 ± 0.04 aAB | 0.82 ± 0.01 bBC | |
| 18 | 1.06 ± 0.07 aA | 1.12 ± 0.04 aA | 0.91 ± 0.13 abA |
| Treatment | Day 0 | Day 7 | Day 14 |
|---|---|---|---|
| Yeasts and molds | |||
| Control | UDL * | 4.0 ± 0.2 **aA | 6.5 ± 0.4 aB |
| 1% ASC | UDL | 5.0 ± 0.0 aA | 6.9 ± 0.2 aB |
| 1% CH–1% ASC | UDL | 2.5 ± 0.3 bA | 3.2 ± 0.2 bA |
| 2% CH–2% ASC | UDL | UDL | UDL |
| 1% CH–5% ASC | UDL | UDL | UDL |
| Aerobic mesophilic bacteria | |||
| Control | UDL | 1.0 ± 0.0 A | 3.1 ± 0.1B |
| 1% ASC | UDL | UDL | UDL |
| 1% CH–1% ASC | UDL | UDL | UDL |
| 2% CH–2% ASC | UDL | UDL | UDL |
| 1% CH–5% ASC | UDL | UDL | UDL |
| Psychrophilic bacteria | |||
| Control | 1.0 ± 0.0 aA | 4.0 ± 0.4 aB | 5.2 ± 0.1 aC |
| 1% ASC | 1.1 ± 0.3 aA | 4.0 ± 0.4 aB | 5.1 ± 0.3 aC |
| 1% CH-1% ASC | 1.0 ± 0.0 aA | 1.9 ± 0.3 bB | UDL |
| 2% CH-2% ASC | 1.2 ± 0.2 a | UDL | UDL |
| 1% CH-5% ASC | UDL | UDL | UDL |
| Population | Treatments | Gompertz Model Parameters | |||
|---|---|---|---|---|---|
| A (log cfu/g) | µmax (Δ log [cfu/g]/day) | λ (day) | R2 | ||
| Yeasts and molds | Control | 6.63 ± 0.46 | 1.16 ± 0.09 | 3.52 ± 0.05 | 1.00 |
| 1% ASC | 6.91 ± 0.20 | 1.38 ± 0.01 | 3.08 ± 0.09 | 1.00 | |
| 1%CH-1%ASC | 2.99 ± 0.42 | 0.49 ± 0.07 | 2.86 ± 0.70 | 1.00 | |
| 2%CH-2%ASC | − | − | − | − | |
| 1%CH-5%ASC | − | − | − | − | |
| Mesophilic aerobic bacteria | Control | 3.32 ± 0.10 | 0.46 ± 0.00 | 4.79 ± 0.02 | 1.00 |
| 1% ASC | − | − | − | − | |
| 1%CH-1%ASC | − | − | − | − | |
| 2%CH-2%ASC | − | − | − | − | |
| 1%CH-5%ASC | − | − | − | − | |
| Psychrophilic bacteria | Control | 4.21 ± 0.11 | 0.94 ± 0.10 | 3.50 ± 0.21 | 1.00 |
| 1% ASC | 3.96 ± 0.04 | 0.94 ± 0.20 | 3.46 ± 0.63 | 1.00 | |
| 1%CH-1%ASC | − | − | − | − | |
| 2%CH-2%ASC | − | − | − | − | |
| 1%CH-5%ASC | − | − | − | − | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özdemir, K.S.; Gökmen, V. Effect of Chitosan-Ascorbic Acid Coatings on the Refrigerated Storage Stability of Fresh-Cut Apples. Coatings 2019, 9, 503. https://doi.org/10.3390/coatings9080503
Özdemir KS, Gökmen V. Effect of Chitosan-Ascorbic Acid Coatings on the Refrigerated Storage Stability of Fresh-Cut Apples. Coatings. 2019; 9(8):503. https://doi.org/10.3390/coatings9080503
Chicago/Turabian StyleÖzdemir, Kübra Sultan, and Vural Gökmen. 2019. "Effect of Chitosan-Ascorbic Acid Coatings on the Refrigerated Storage Stability of Fresh-Cut Apples" Coatings 9, no. 8: 503. https://doi.org/10.3390/coatings9080503