Surface Modification of Hemoglobin-Based Oxygen Carriers Reduces Recognition by Haptoglobin, Immunoglobulin, and Hemoglobin Antibodies
Abstract
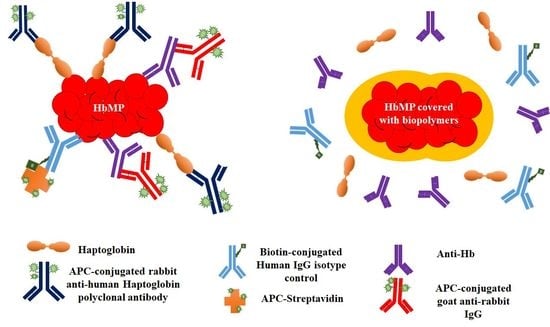
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Particle Preparation
2.2.1. Preparation of Hemoglobin Submicron Particles (HbMPs)
2.2.2. Preparation of HSA-d-HbMPs and HSA-t-HbMPs
2.2.3. Preparation of HA-d-HbMPs and HA-t-HbMPs
2.2.4. Preparation of Plu-d-HbMPs and Plu-t-HbMPs
2.3. Particle Characterization
2.3.1. Size and Zeta-Potential
2.3.2. Transmission Electron Microscopy (TEM)
2.3.3. Confocal Laser Scanning Microscopy (CLSM)
2.3.4. Determination of Hb Content
2.3.5. Determination of Oxygenated HbMPs (Oxy-Hbs)
2.4. In Vitro Antibody Binding Assays
2.4.1. Binding Assay of Anti-Hb Antibodies
2.4.2. Binding Assay of Immunoglobulin (IgG)
2.4.3. Binding Assay of Haptoglobin (HP)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Particle Characterization
3.1.1. Particle Morphology and Size
3.1.2. Zeta Potential
3.1.3. Hemoglobin Content and Oxygen Binding Capacity
3.2. Binding of Anti-Hb Antibodies to Surface-Modified HbMPs
3.3. Binding of IgG to Surface-Modified HbMPs
3.4. Binding of Haptoglobin to Surface-Modified HbMPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, S.; Sharma, P.; Tyler, L.N. Transfusion of blood and blood products: Indications and complications. Am. Fam. Physician 2011, 83, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Rawn, J. The silent risks of blood transfusion. Curr. Opin. Anaesthesiol. 2008, 21, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Jahanian-Najafabadi, A.; Roudkenar, M.H. Artificial Blood Substitutes: First steps on the long route to clinical utility. Clin. Med. Insights Blood Disord. 2016, 9, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Seifried, E.; Mueller, M.M. The present and future of transfusion medicine. Blood Transfus. 2011, 9, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Winslow, R.M. Current status of oxygen carriers. Vox Sang. 2006, 91, 102–110. [Google Scholar] [CrossRef]
- Keipert, P.E. Hemoglobin-Based Oxygen Carrier (HBOC) Development in Trauma: Previous Regulatory Challenges, Lessons Learned, and a Path Forward; Advances in Experimental Medicine and Biology Series 977; Springer: Cham, Switzerland, 2017; pp. 343–350. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Scerbo, M.; Kramer, G. A review of blood substitutes: Examining the history, clinical trial results, and ethics of hemoglobin-based oxygen carriers. Clinics 2009, 64, 803–814. [Google Scholar] [CrossRef]
- Alayash, A.I. Blood substitutes: Why haven’t we been more successful? Trends Biotechnol. 2014, 32, 177–185. [Google Scholar] [CrossRef]
- Jia, Y.; Duan, L.; Li, J. Hemoglobin-based nanoarchitectonic assemblies as oxygen carriers. Adv. Mater. 2016, 28, 1312–1318. [Google Scholar] [CrossRef]
- Estep, T.N. Pharmacokinetics and mechanisms of plasma removal of hemoglobin-based oxygen carriers. Artif. Cells Nanomed. Biotechnol. 2015, 43, 203–215. [Google Scholar] [CrossRef]
- Shih, A.W.Y.; Mcfarlane, A.; Verhovsek, M. Haptoglobin testing in hemolysis: Measurement and interpretation. Am. J. Hematol. 2014, 89, 443–447. [Google Scholar] [CrossRef]
- Schaer, D.J.; Vinchi, F.; Ingoglia, G.; Tolosano, E.; Buehler, P.W. Haptoglobin, hemopexin, and related defense pathways—basic science, clinical perspectives, and drug development. Front. Physiol. 2014, 5, 415. [Google Scholar] [CrossRef] [PubMed]
- Salmaso, S.; Caliceti, P. Stealth properties to improve therapeutic efficacy of drug nanocarriers. J. Drug Deliv. 2013, 2013, 374252. [Google Scholar] [CrossRef] [PubMed]
- Amoozgar, Z.; Yeo, Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 219–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, B.F.; Blank, L.M.; Mclaughlin, R.; Nielsen, L.K. Microbial hyaluronic acid production. Appl. Microbiol. Biotechnol. 2005, 66, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Szarpak, A.; Cui, D.; Dubreuil, F.; De Geest, B.G.; De Cock, L.J.; Picart, C.; Auzély-Velty, R. Designing hyaluronic acid-based layer-by-layer capsules as a carrier for intracellular drug delivery. Biomacromolecules 2010, 11, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Gyulai, G.; Magyar, A.; Rohonczy, J.; Orosz, J.; Yamasaki, M.; Bősze, S.; Kiss, E. Preparation and characterization of cationic pluronic for surface modification and functionalization of polymeric drug delivery nanoparticles. Express Polym. Lett. 2016, 10, 216–226. [Google Scholar] [CrossRef]
- Nitta, S.K.; Numata, K. Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int. J. Mol. Sci. 2013, 14, 1629–1654. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Mu, J.; Xing, B. Recent advances on the development of pharmacotherapeutic agents on the basis of human serum albumin. Curr. Pharm. Des. 2015, 21, 1866–1888. [Google Scholar] [CrossRef]
- Esfandyari-manesh, M.; Mostafavi, S.H.; Majidi, R.F.; Koopaei, M.N.; Ravari, N.S.; Amini, M.; Darvishi, B.; Ostad, S.N.; Atyabi, F.; Dinarvand, R. Improved anticancer delivery of paclitaxel by albumin surface modification of PLGA nanoparticles. DARU J. Pharm. Sci. 2015, 23, 28. [Google Scholar] [CrossRef]
- Bäumler, H.; Xiong, Y.; Liu, Z.Z.; Patzak, A.; Georgieva, R. Novel hemoglobin particles-promising new-generation hemoglobin-based oxygen carriers. Artif. Organs 2014, 38, 708–714. [Google Scholar] [CrossRef]
- Kao, I.; Xiong, Y.; Steffen, A.; Smuda, K.; Zhao, L.; Georgieva, R.; Pruss, A.; Bäumler, H. Preclinical in vitro safety investigations of submicron sized hemoglobin based oxygen carrier HbMP-700. Artif. Organs 2018, 42, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Haney, C.R.; Buehler, P.W.; Gulati, A. Purification and chemical modifications of hemoglobin in developing hemoglobin based oxygen carriers. Adv. Drug Deliv. Rev. 2000, 40, 153–169. [Google Scholar] [CrossRef]
- Bäumler, H.; Georgieva, R. Coupled enzyme reactions in multicompartment microparticles. Biomacromolecules 2010, 11, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Steffen, A.; Andreas, K.; Müller, S.; Sternberg, N.; Georgieva, R.; Bäumler, H. Hemoglobin-based oxygen carrier microparticles: Synthesis, properties, and in vitro and in vivo investigations. Biomacromolecules 2012, 13, 3292–3300. [Google Scholar] [CrossRef] [PubMed]
- Kloypan, C.; Prapan, A.; Suwannasom, N.; Chaiwaree, S.; Kaewprayoon, W.; Steffen, A.; Xiong, Y.; Baisaeng, N.; Georgieva, R.; Bäumler, H. Improved oxygen storage capacity of haemoglobin submicron particles by one-pot formulation. Artif. Cells Nanomed. Biotechnol. 2018, 46, S964–S972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anchinmane, V.T.; Sankhe, S.V. Evaluation of hemoglobin estimation with non-cyanide alkaline haematin D-575 method. Int. J. Res. Med. Sci. 2016, 44, 4297–4299. [Google Scholar] [CrossRef]
- Haldane, J. The ferricyanide method of determining the oxygen capacity of blood. J Physiol. 1900, 25, 295–302. [Google Scholar] [CrossRef]
- Baidukova, O.; Wang, Q.; Chaiwaree, S.; Freyer, D.; Prapan, A.; Georgieva, R.; Zhao, L.; Baumler, H. Antioxidative protection of haemoglobin microparticles (HbMPs) by polydopamine. Artif. Cells Nanomed. Biotechnol. 2018, 46, S693–S701. [Google Scholar] [CrossRef]
- Xiong, Y.; Georgieva, R.; Steffen, A.; Smuda, K.; Bäumler, H. Structure and properties of hybrid biopolymer particles fabricated by co-precipitation cross-linking dissolution procedure. J. Colloid Interface Sci. 2018, 514, 156–164. [Google Scholar] [CrossRef]
- Cook, S.F. The action of potassium cyanide on certain respiratory pigments. J. Gen. Physiol. 1928, 11, 339–348. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Yuan, Y.; Shan, X.; Sheng, Y.; Xu, F. A noninvasive method for measuring the oxygen binding-releasing capacity of hemoglobin-loaded polymeric nanoparticles as oxygen carrier. J. Mater. Sci. Mater. Med. 2009, 20, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Feng, K.; Li, Q.; Ma, H.; Zhu, H.; Xie, Y.; Yan, G.; Chen, C.; Yan, K. Glutaraldehyde-polymerized hemoglobin and tempol (PolyHb-Tempol) has superoxide dismutase activity that can attenuate oxidative stress on endothelial cells induced by superoxide anion. Artif. Cells Nanomed. Biotechnol. 2018, 46, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wood, F.; Buehler, P.W.; Alayash, A.I. Haptoglobin preferentially binds β but not α subunits cross-linked hemoglobin tetramers with minimal effects on ligand and redox reactions. PLoS ONE 2013, 8, e59841. [Google Scholar] [CrossRef] [PubMed]
- Pigman, W.; Gramling, E.; Holley, H.L. Interactions of hyaluronic acid with serum albumin. Biochim. Biophys. Acta 1961, 46, 100–107. [Google Scholar] [CrossRef]
- Neacsu, M.V.; Matei, I.; Micutz, M.; Staicu, T.; Precupas, A.; Popa, V.T.; Salifoglou, A.; Ionita, G. Interaction between albumin and pluronic F127 block copolymer revealed by global and local physicochemical profiling. J. Phys. Chem. B 2016, 120, 4258–4267. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Liu, Z.Z.; Georgieva, R.; Smuda, K.; Steffen, A.; Sendeski, M.; Voigt, A.; Patzak, A.; Bäumler, H. Nonvasoconstrictive hemoglobin particles as oxygen carriers. ACS Nano 2013, 7, 7454–7461. [Google Scholar] [CrossRef] [PubMed]
- Alayash, A.I. Mechanisms of toxicity and modulation of hemoglobin-based oxygen carriers (HBOCs). Shock 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Toma, V.A.; Farca, A.D.; Roman, I.; Sevastre, B.; Hathazi, D.; Scurtu, F.; Damian, G.; Silaghi-Dumitrescu, R. Comparative in vivo effects of hemoglobin- based oxygen carriers ( HBOC ) with varying prooxidant and physiological reactivity. PLoS ONE 2016, 11, e0153909. [Google Scholar] [CrossRef]







| Particle Type | Abbreviation |
|---|---|
| Hemoglobin submicron particles | HbMPs |
| Double precipitated HbMP in presence of HSA: Hb precipitation first, HSA absorption before second precipitation step. | HSA-d-HbMP |
| Double precipitated HbMP in presence of HA: Hb precipitation first, HA and HSA absorption before second precipitation step. | HA-d-HbMP |
| Double precipitated HbMP in presence of pluronic: Hb precipitation first, Plu and HSA absorption before second precipitation step. | Plu-d-HbMP |
| Triple precipitated HbMP in presence of HSA: Hb precipitation first, HSA absorption before second and third precipitation step. | HSA-t-HbMP |
| Triple precipitated HbMP in presence of HA: Hb precipitation first, HA and HSA absorption before second and third precipitation step. | HA-t-HbMP |
| Triple precipitated HbMP in presence of pluronic: Hb precipitation first, Plu and HSA absorption before second and third precipitation step. | Plu-t-HbMP |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prapan, A.; Suwannasom, N.; Kloypan, C.; Chaiwaree, S.; Steffen, A.; Xiong, Y.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Surface Modification of Hemoglobin-Based Oxygen Carriers Reduces Recognition by Haptoglobin, Immunoglobulin, and Hemoglobin Antibodies. Coatings 2019, 9, 454. https://doi.org/10.3390/coatings9070454
Prapan A, Suwannasom N, Kloypan C, Chaiwaree S, Steffen A, Xiong Y, Kao I, Pruß A, Georgieva R, Bäumler H. Surface Modification of Hemoglobin-Based Oxygen Carriers Reduces Recognition by Haptoglobin, Immunoglobulin, and Hemoglobin Antibodies. Coatings. 2019; 9(7):454. https://doi.org/10.3390/coatings9070454
Chicago/Turabian StylePrapan, Ausanai, Nittiya Suwannasom, Chiraphat Kloypan, Saranya Chaiwaree, Axel Steffen, Yu Xiong, Ijad Kao, Axel Pruß, Radostina Georgieva, and Hans Bäumler. 2019. "Surface Modification of Hemoglobin-Based Oxygen Carriers Reduces Recognition by Haptoglobin, Immunoglobulin, and Hemoglobin Antibodies" Coatings 9, no. 7: 454. https://doi.org/10.3390/coatings9070454