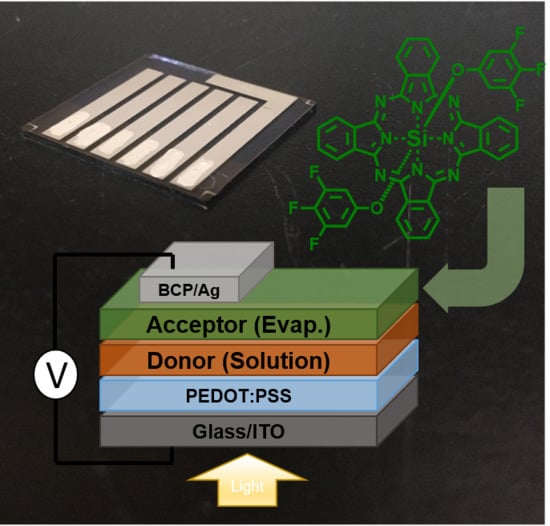
Silicon Phthalocyanines as Acceptor Candidates in Mixed Solution/Evaporation Processed Planar Heterojunction Organic Photovoltaic Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Device Fabrication
2.3. Device Characterization
3. Results and Discussion
3.1. Determination of the Acceptor/Donor Couple
3.2. (345F)2-SiPc Thickness Improvement
3.3. Thermal Treatment
3.1.1. Annealing before the Evaporation of (345F)2-SiPc
3.1.2. Annealing after the (345F)2-SiPc Evaporation
3.1.3. Annealing during the (345F)2-SiPc Evaporation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Battaglia, C.; Cuevas, A.; De Wolf, S. High-efficiency crystalline silicon solar cells: status and perspectives. Energy Environ. Sci. 2016, 9, 1552–1576. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, H.; Sariciftci, N.S. Organic solar cells: An overview. J. Mater. Res. 2004, 19, 1924–1945. [Google Scholar] [CrossRef]
- Forrest, S.R. The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 2004, 428, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, L.H.; Andreasen, J.B.; Marquard, M.; Debois, S.; Larsen, F.S.; Jeppesen, V. Declarative process models in government centric case and document management. In Proceedings of the BPM 2017 Industry Track, Barcelona, Spain, 10–15 September 2017. [Google Scholar]
- Dang, M.T.; Hirsch, L.; Wantz, G. P3HT:PCBM, best seller in polymer photovoltaic research. Adv. Mater. 2011, 23, 3597–3602. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhang, Y.; Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.; Lau, T.; Lu, X. Single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core. Joule 2019. [Google Scholar] [CrossRef]
- Yan, C.; Barlow, S.; Wang, Z.; Yan, H.; Jen, A.K.Y.; Marder, S.R.; Zhan, X. Non-fullerene acceptors for organic solar cells. Nat. Rev. Mater. 2018, 3, 18003. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, B.H.; Su, Y.W.; Jeng, R.J.; Wang, Y.J.; Chen, C.P.; Wong, K.T. Si-bridged ladder-type small-molecule acceptors for high-performance organic photovoltaics. ACS Appl. Mater. Interfaces 2018, 11, 1125–1134. [Google Scholar] [CrossRef]
- Yu, Y.; Tsai, T.; Chen, C. Efficient ternary organic photovoltaics using two conjugated polymers and a nonfullerene acceptor with complementary absorption and cascade energy-level alignment. J. Phys. Chem. C 2018, 122, 24585–24591. [Google Scholar] [CrossRef]
- Schaffer, C.J.; Palumbiny, C.M.; Niedermeier, M.A.; Jendrzejewski, C.; Santoro, G.; Roth, S.V.; Mller-Buschbaum, P. A direct evidence of morphological degradation on a nanometer scale in polymer solar cells. Adv. Mater. 2013, 25, 6760–6764. [Google Scholar] [CrossRef] [PubMed]
- Ayzner, A.L.; Tassone, C.J.; Tolbert, S.H.; Schwartz, B.J. Reappraising the need for bulk heterojunctions in polymer-fullerene photovoltaics: The role of carrier transport in all-solution-processed P3HT/PCBM bilayer solar cells. J. Phys. Chem. C 2009, 113, 20050–20060. [Google Scholar] [CrossRef]
- Lee, K.H.; Schwenn, P.E.; Smith, A.R.G.; Cavaye, H.; Shaw, P.E.; James, M.; Krueger, K.B.; Gentle, I.R.; Meredith, P.; Burn, P.L. Morphology of all-solution-processed “bilayer” organic solar cells. Adv. Mater. 2011, 23, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Vohra, V.; Arrighetti, G.; Barba, L.; Higashimine, K.; Porzio, W.; Murata, H. Enhanced vertical concentration gradient in rubbed P3HT:PCBM graded bilayer solar cells. J. Phys. Chem. Lett. 2012, 3, 1820–1823. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.S.; Takacs, C.J.; Sun, Y.; Heeger, A.J. Spontaneous formation of bulk heterojunction nanostructures: Multiple routes to equivalent morphologies. Nano Lett. 2011, 11, 1036–1039. [Google Scholar] [CrossRef]
- Gholamkhass, B.; Servati, P. Solvent-vapor induced morphology reconstruction for efficient PCDTBT based polymer solar cells. Org. Electron. Phys. Mater. Appl. 2013, 14, 2278–2283. [Google Scholar] [CrossRef]
- Wakim, S.; Beaupré, S.; Blouin, N.; Aich, B.; Rodman, S.; Gaudiana, R.; Tao, Y.; Leclerc, M. Highly efficient organic solar cells based on a poly(2,7-carbazole) derivative. J. Mater. Chem. 2009, 19, 5351–5358. [Google Scholar] [CrossRef]
- Park, S.H.; Roy, A.; Beaupré, S.; Cho, S.; Coates, N.; Moon, J.S.; Moses, D.; Leclerc, M.; Lee, K.; Heeger, A.J. Bulk heterojunction solar cells with internal quantum efficiency approaching 100%. Nat. Photonics 2009, 3, 297–303. [Google Scholar] [CrossRef]
- Wang, D.H.; Kim, J.K.; Seo, J.H.; Park, O.O.; Park, J.H. Stability comparison: A PCDTBT/PC 71BM bulk-heterojunction versus a P3HT/PC 71BM bulk-heterojunction. Sol. Energy Mater. Sol. Cells 2012, 101, 249–255. [Google Scholar] [CrossRef]
- Seok, J.; Shin, T.J.; Park, S.; Cho, C.; Lee, J.Y.; Ryu, D.Y.; Kim, M.H.; Kim, K. Efficient Organic Photovoltaics Utilizing Nanoscale Heterojunctions in Sequentially Deposited Polymer/fullerene Bilayer. Sci. Rep. 2015, 5, 8373. [Google Scholar] [CrossRef] [Green Version]
- Josey, D.S.; Castrucci, J.S.; Dang, J.D.; Lessard, B.H.; Bender, T.P. Evaluating thiophene electron-donor layers for the rapid assessment of boron subphthalocyanines as electron acceptors in organic photovoltaics: Solution or vacuum deposition? ChemPhysChem 2015, 16, 1245–1250. [Google Scholar] [CrossRef]
- Yang, Y.; Aryal, M.; Mielczarek, K.; Hu, W.; Zakhidov, A. Nanoimprinted P3HT/C60 solar cells optimized by oblique deposition of C60. J. Vac. Sci. Technol. B 2010, 28, C6M104–C6M107. [Google Scholar]
- Li, C.Z.; Yip, H.L.; Jen, A.K.Y. Functional fullerenes for organic photovoltaics. J. Mater. Chem. 2012, 22, 4161–4177. [Google Scholar] [CrossRef]
- Hoke, E.T.; Vandewal, K.; Bartelt, J.A.; Mateker, W.R.; Douglas, J.D.; Noriega, R.; Graham, K.R.; Fréchet, J.M.J.; Salleo, A.; McGehee, M.D. Recombination in polymer:Fullerene solar cells with open-circuit voltages approaching and exceeding 1.0 V. Adv. Energy Mater. 2013, 3, 220–230. [Google Scholar] [CrossRef]
- Kooistra, F.B.; Knol, J.; Kastenberg, F.; Popescu, L.M.; Verhees, W.J.H.; Kroon, J.M.; Hummelen, J.C. Increasing the open circuit voltage of bulk-heterojunction solar cells by raising the LUMO level of the acceptor. Org. Lett. 2007, 9, 551–554. [Google Scholar] [CrossRef]
- Anctil, A.; Babbitt, C.W.; Raffaelle, R.P.; Landi, B.J. Material and energy intensity of fullerene production. Environ. Sci. Technol. 2011, 45, 2353–2359. [Google Scholar] [CrossRef]
- Anctil, A.; Babbitt, C.W.; Raffaelle, R.P.; Landi, B.J. Cumulative energy demand for small molecule and polymer photovoltaics. Prog. Photovol. Res. Appl. 2013, 21, 1541–1554. [Google Scholar] [CrossRef]
- Allen, C.G.; Martin, I.; Gordon, K. Hydroxygallium phthalocyanine pigments with block copolymer binders. U.S. Patent 5521043, 28 May 1996. [Google Scholar]
- Giambalvo, V.A.; Lee, W.; Cyan, A. Non-crystallizing, non-flocculating phthalocyanines. U.S. Patent 3589924, 29 June 1971. [Google Scholar]
- De La Torre, G.; Claessens, C.G.; Torres, T. Phthalocyanines: Old dyes, new materials. Putting color in nanotechnology. Chem. Commun. 2007, 20, 2000–2015. [Google Scholar] [CrossRef]
- Lim, B.; Margulis, G.Y.; Yum, J.; Unger, E.L.; Hardin, B.E.; Gr, M.; Mcgehee, M.D.; Sellinger, A. Silicon-naphthalo/phthalocyanine-hybrid sensitizer for efficient red response in dye-sensitized solar cells. Org. Lett. 2013, 15, 2011–2014. [Google Scholar] [CrossRef]
- El-nahass, M.M.; El-gohary, Z.; Soliman, H.S. Structural and optical studies of thermally evaporated CoPc thin films. Opt. Laser Technol. 2003, 35, 523–531. [Google Scholar] [CrossRef]
- Roberts, M.E.; Sokolov, A.N.; Bao, Z. Material and device considerations for organic thin-film transistor sensors. J. Mater. Chem. 2009, 19, 3351–3363. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Xie, G.; Tai, H.; Sun, P.; Zhang, B. Copper phthalocyanine thin film transistors for hydrogen sulfide detection. Sens. Actuators B Chem. 2013, 176, 1191–1196. [Google Scholar] [CrossRef]
- Melville, O.A.; Lessard, B.H.; Bender, T.P. Phthalocyanine-based organic thin-film transistors: A review of recent advances. ACS Appl. Mater. Interfaces 2015, 7, 13105–13118. [Google Scholar] [CrossRef]
- Boileau, N.T.; Melville, O.A.; Mirka, B.; Cranston, R.; Lessard, B.H. P and N type copper phthalocyanines as effective semiconductors in organic thin-film transistor based DNA biosensors at elevated temperatures. RSC Adv. 2019, 9, 2133–2142. [Google Scholar] [CrossRef]
- Melville, O.A.; Grant, T.M.; Lessard, B.H. Silicon phthalocyanines as N-type semiconductors in organic thin film transistors. J. Mater. Chem. C 2018, 6, 5482–5488. [Google Scholar] [CrossRef]
- Deng, Z.; Lü, Z.; Chen, Y.; Yin, Y.; Zou, Y.; Xiao, J.; Wang, Y. Aluminum phthalocyanine chloride as a hole injection enhancer in organic light-emitting diodes. Solid-State Electron. 2013, 89, 22–25. [Google Scholar] [CrossRef]
- Lessard, B.H.; Sampson, K.L.; Plint, T.; Bender, T.P. Boron subphthalocyanine polymers: Avoiding the small molecule side product and exploring their use in organic light-emitting diodes. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1996–2006. [Google Scholar] [CrossRef]
- Pearson, A.J.; Plint, T.; Jones, S.T.; Lessard, B.H.; Credgington, D.; Bender, T.P.; Greenham, N.C. Silicon phthalocyanines as dopant red emitters for efficient solution processed OLEDs. J. Mater. Chem. C 2017, 5, 12688–12698. [Google Scholar] [CrossRef]
- Plint, T.; Lessard, B.H.; Bender, T.P. Assessing the potential of group 13 and 14 metal/metalloid phthalocyanines as hole transport layers in organic light emitting diodes. J. Appl. Phys. 2016, 119, 145502. [Google Scholar] [CrossRef]
- Xue, J.; Uchida, S.; Rand, B.P.; Forrest, S.R. Asymmetric tandem organic photovoltaic cells with hybrid planar-mixed molecular heterojunctions. Appl. Phys. Lett. 2004, 85, 5757–5759. [Google Scholar] [CrossRef]
- Kim, D.Y.; So, F.; Gao, Y. Aluminum phthalocyanine chloride/C60 organic photovoltaic cells with high open-circuit voltages. Sol. Energy Mater. Sol. Cells 2009, 93, 1688–1691. [Google Scholar] [CrossRef]
- Cnops, K.; Rand, B.P.; Cheyns, D.; Verreet, B.; Empl, M.A.; Heremans, P. 8.4% efficient fullerene-free organic solar cells exploiting long-range exciton energy transfer. Nat. Commun. 2014, 5, 3406. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.T.; Grant, T.M.; Yan, H.; Seferos, D.S.; Lessard, B.H.; Bender, T.P. Bis(tri-n-alkylsilyl oxide) silicon phthalocyanines: A start to establishing a structure property relationship as both ternary additives and non-fullerene electron acceptors in bulk heterojunction organic photovoltaic devices. J. Mater. Chem. A 2017, 5, 12168–12182. [Google Scholar] [CrossRef]
- Yuen, A.P.; Jovanovic, S.M.; Hor, A.M.; Klenkler, R.A.; Devenyi, G.A.; Loutfy, R.O.; Preston, J.S. Photovoltaic properties of M-phthalocyanine/fullerene organic solar cells. Sol. Energy 2012, 86, 1683–1688. [Google Scholar] [CrossRef]
- Williams, G.; Sutty, S.; Klenkler, R.; Aziz, H. Renewed interest in metal phthalocyanine donors for small molecule organic solar cells. Sol. Energy Mater. Sol. Cells 2014, 124, 217–226. [Google Scholar] [CrossRef]
- Lessard, B.H.; Dang, J.D.; Grant, T.M.; Gao, D.; Seferos, D.S.; Bender, T.P. Bis(tri-n-hexylsilyl oxide) silicon phthalocyanine: A unique additive in ternary bulk heterojunction organic photovoltaic devices. ACS Appl. Mater. Interfaces 2014, 6, 15040–15051. [Google Scholar] [CrossRef] [PubMed]
- Grant, T.M.; Gorisse, T.; Dautel, O.; Wantz, G.; Lessard, B.H. Multifunctional ternary additive in bulk heterojunction OPV: Increased device performance and stability. J. Mater. Chem. A 2017, 5, 1581–1587. [Google Scholar] [CrossRef]
- Honda, S.; Ohkita, H.; Benten, H.; Ito, S. Multi-colored dye sensitization of polymer/fullerene bulk heterojunction solar cells. Chem. Commun. 2010, 46, 6596–6598. [Google Scholar] [CrossRef]
- Honda, S.; Ohkita, H.; Benten, H.; Ito, S. Selective dye loading at the heterojunction in polymer/fullerene solar cells. Adv. Energy Mater. 2011, 1, 588–598. [Google Scholar] [CrossRef]
- Lessard, B.H.; White, R.T.; Al-Amar, M.; Plint, T.; Castrucci, J.S.; Josey, D.S.; Lu, Z.H.; Bender, T.P. Assessing the potential roles of silicon and germanium phthalocyanines in planar heterojunction organic photovoltaic devices and how pentafluoro phenoxylation can enhance π-π Interactions and device performance. ACS Appl. Mater. Interfaces 2015, 7, 5076–5088. [Google Scholar] [CrossRef]
- Lessard, B.H.; Grant, T.M.; White, R.; Thibau, E.; Lu, Z.H.; Bender, T.P. The position and frequency of fluorine atoms changes the electron donor/acceptor properties of fluorophenoxy silicon phthalocyanines within organic photovoltaic devices. J. Mater. Chem. A 2015, 3, 24512–24524. [Google Scholar] [CrossRef]
- Zysman-Colman, E.; Ghosh, S.S.; Xie, G.; Varghese, S.; Chowdhury, M.; Sharma, N.; Cordes, D.B.; Slawin, A.M.Z.; Samuel, I.D.W. Solution-processable silicon phthalocyanines in electroluminescent and photovoltaic devices. ACS Appl. Mater. Interfaces 2016, 8, 9247–9253. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, K.V.; Sullivan, P.; Yang, J.L.; Jones, T.S. Efficient organic photovoltaic cells through structural modification of chloroaluminum phthalocyanine/fullerene heterojunctions. J. Phys. Chem. C 2010, 114, 3304–3308. [Google Scholar] [CrossRef]
- Lessard, B.H.; Al-Amar, M.; Grant, T.M.; White, R.; Lu, Z.H.; Bender, T.P. From chloro to fluoro, expanding the role of aluminum phthalocyanine in organic photovoltaic devices. J. Mater. Chem. A 2015, 3, 5047–5053. [Google Scholar] [CrossRef]
- Bailey-Salzman, R.F.; Rand, B.P.; Forrest, S.R. Near-infrared sensitive small molecule organic photovoltaic cells based on chloroaluminum phthalocyanine. Appl. Phys. Lett. 2007, 91, 1–4. [Google Scholar] [CrossRef]
- Chen, W.B.; Xiang, H.F.; Xu, Z.X.; Yan, B.P.; Roy, V.A.L.; Che, C.M.; Lai, P.T. Improving efficiency of organic photovoltaic cells with pentacene-doped CuPc layer. Appl. Phys. Lett. 2007, 91, 191109. [Google Scholar] [CrossRef] [Green Version]
- Kumar, H.; Kumar, P.; Bhardwaj, R.; Sharma, G.D.; Chand, S.; Jain, S.C.; Kumar, V. Broad spectral sensitivity and improved efficiency in CuPc/Sub-Pc organic photovoltaic devices. J. Phys. D Appl. Phys. 2008, 42, 015103. [Google Scholar] [CrossRef]
- Brumbach, M.; Placencia, D.; Armstrong, N.R. Titanyl phthalocyanine/C60 heterojunctions: Band-edge offsets and photovoltaic device performance. J. Phys. Chem. C 2008, 112, 3142–3151. [Google Scholar] [CrossRef]
- Yu, J.; Huang, J.; Zhang, L.; Jiang, Y. Energy losing rate and open-circuit voltage analysis of organic solar cells based on detailed photocurrent simulation. J. Appl. Phys. 2009, 106, 063103. [Google Scholar]
- Li, N.; Lassiter, B.E.; Lunt, R.R.; Wei, G.; Forrest, S.R. Open circuit voltage enhancement due to reduced dark current in small molecule photovoltaic cells Open circuit voltage enhancement due to reduced dark current in small molecule photovoltaic cells. Appl. Phys. Lett. 2009, 94, 023307. [Google Scholar]
- Du, C.; Yu, J.; Huang, J.; Jiang, Y. Organic solar cells using Tin (II) phthalocyanine as donor material. Energy Procedia 2011, 12, 519–524. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Hoppe, H.; Erb, T.; Günes, S.; Gobsch, G.; Sariciftci, N.S. Effects of annealing on the nanomorphology and performance of poly(alkylthiophene):Fullerene bulk-heterojunction solar cells. Adv. Funct. Mater. 2007, 17, 1071–1078. [Google Scholar] [CrossRef]





| Sample a | # of Devices | Jsc (mA∙cm−2) | Voc (V) | FF | ηeff (%) |
|---|---|---|---|---|---|
| P3HT:PC61BM a | 84 | 8.2 ± 0.4 | 0.56 ± 0.02 | 0.61 ± 0.04 | 2.87 ± 0.21 |
| P3HT/C60 (40 nm) | 8 | 2.58 ± 0.05 | 0.22 ± 0.01 | 0.48 ± 0.02 | 0.28 ± 0.02 |
| PCDTBT/C60 (40 nm) | 9 | 4.7 ± 0.1 | 0.56 ± 0.01 | 0.57 ± 0.02 | 1.48 ± 0.06 |
| P3HT/(345F)2-SiPc (60 nm) | 9 | 1.09 ± 0.06 | 0.34 ± 0.03 | 0.48 ± 0.07 | 0.19 ± 0.04 |
| P3HT/(246F)2-SiPc (40 nm) | 6 | 0.62 ± 0.06 | 0.49 ± 0.02 | 0.47 ± 0.04 | 0.14 ± 0.09 |
| PCDTBT/(246F)2-SiPc (20 nm) | 5 | 0.47 ± 0.03 | 0.07 ± 0.02 | 0.25 ± 0.01 | 0.01 ± 0.004 |
| PCDTBT/(345F)2-SiPc (93 nm) | 9 | 3.3 ± 0.1 | 0.87 ± 0.03 | 0.33 ± 0.01 | 0.97 ± 0.06 |
| PCDTBT/(345F)2-SiPc (93 nm) b | 7 | 3.4 ± 0.2 | 0.88 ± 0.02 | 0.51 ± 0.02 | 1.52 ± 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faure, M.D.M.; Grant, T.M.; Lessard, B.H. Silicon Phthalocyanines as Acceptor Candidates in Mixed Solution/Evaporation Processed Planar Heterojunction Organic Photovoltaic Devices. Coatings 2019, 9, 203. https://doi.org/10.3390/coatings9030203
Faure MDM, Grant TM, Lessard BH. Silicon Phthalocyanines as Acceptor Candidates in Mixed Solution/Evaporation Processed Planar Heterojunction Organic Photovoltaic Devices. Coatings. 2019; 9(3):203. https://doi.org/10.3390/coatings9030203
Chicago/Turabian StyleFaure, Marie D. M., Trevor M. Grant, and Benoît H. Lessard. 2019. "Silicon Phthalocyanines as Acceptor Candidates in Mixed Solution/Evaporation Processed Planar Heterojunction Organic Photovoltaic Devices" Coatings 9, no. 3: 203. https://doi.org/10.3390/coatings9030203