Toward Superhydrophobic/Superoleophilic Materials for Separation of Oil/Water Mixtures and Water-in-Oil Emulsions Using Phase Inversion Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
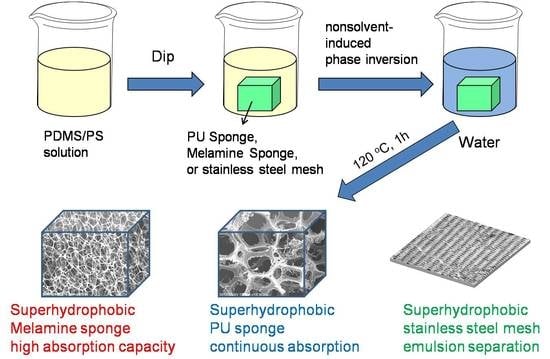
2.2. Preparation of PDMS/PS Coated Substrates
2.3. Water-in-Oil Emulsions
2.4. Water-in-Oil Emulsions Separation Experiment
2.5. Instruments and Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dalton, T.; Jin, D. Extent and Frequency of Vessel Oil Spills in Us Marine Protected Areas. Mar. Pollut. Bull. 2010, 60, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Yip, T.L.; Talley, W.K.; Jin, D. The Effectiveness of Double Hulls in Reducing Vessel-Accident Oil Spillage. Mar. Pollut. Bull. 2011, 62, 2427–2432. [Google Scholar] [CrossRef] [PubMed]
- Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T.; Carmody, O.; Kokot, S. Porous Materials for Oil Spill Cleanup: A Review of Synthesis and Absorbing Properties. J. Porous Mater. 2003, 10, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Bayat, A.; Aghamiri, S.F.; Moheb, A.; Vakili-Nezhaad, G.R. Oil Spill Cleanup from Sea Water by Sorbent Materials. Chem. Eng. Technol. 2005, 28, 1525–1528. [Google Scholar] [CrossRef]
- Sayari, A.; Hamoudi, S.; Yang, Y. Applications of Pore-Expanded Mesoporous Silica. 1. Removal of Heavy Metal Cations and Organic Pollutants from Wastewater. Chem. Mater. 2005, 17, 212–216. [Google Scholar] [CrossRef]
- Ono, T.; Sugimoto, T.; Shinkai, S.; Sada, K. Molecular Design of Superabsorbent Polymers for Organic Solvents by Crosslinked Lipophilic Polyelectrolytes. Adv. Funct. Mater. 2008, 18, 3936–3940. [Google Scholar] [CrossRef]
- Li, Z.T.; Lin, B.; Jiang, L.W.; Lin, E.C.; Chen, J.; Zhang, S.J.; Tang, Y.W.; He, F.A.; Li, D.H. Effective Preparation of Magnetic Superhydrophobic Fe3o4/Pu Sponge for Oil-Water Separation. Appl. Surf. Sci. 2018, 427, 56–64. [Google Scholar] [CrossRef]
- Zhang, X.; Zhi, D.F.; Zhu, W.Z.; Sathasivam, S.; Parkin, I.P. Facile Fabrication of Durable Superhydrophobic Sio2/Polyurethane Composite Sponge for Continuous Separation of Oil from Water. RSC Adv. 2017, 7, 11362–11366. [Google Scholar] [CrossRef]
- Gao, J.F.; Song, X.; Huang, X.W.; Wang, L.; Li, B.; Xue, H.G. Facile Preparation of Polymer Microspheres and Fibers with a Hollow Core and Porous Shell for Oil Adsorption and Oil/Water Separation. Appl. Surf. Sci. 2018, 439, 394–404. [Google Scholar] [CrossRef]
- Tran, V.H.T.; Lee, B.K. Novel Fabrication of a Robust Superhydrophobic Pu@Zno@Fe3o4@Sa Sponge and Its Application in Oil-Water Separations. Sci. Rep. 2017, 7, 17520. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Yang, B.; Barras, A.; Szunerits, S.; Boukherroub, R. Polyurethane Sponge Functionalized with Superhydrophobic Nanodiamond Particles for Efficient Oil/Water Separation. Chem. Eng. J. 2017, 307, 319–325. [Google Scholar] [CrossRef]
- Wang, Y.K.; Wang, B.; Wang, J.H.; Ren, Y.F.; Xuan, C.Y.; Liu, C.T.; Shen, C.Y. Superhydrophobic and Superoleophilic Porous Reduced Graphene Oxide/Polycarbonate Monoliths for High-Efficiency Oil/Water Separation. J. Hazard. Mater. 2018, 344, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.T.; Su, P.K.; Hu, C.C.; Lai, J.Y.; Liu, Y.L. Surface Modification of Porous Substrates for Oil/Water Separation Using Crosslinkable Polybenzoxazine as an Agent. J. Membr. Sci. 2018, 546, 100–109. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.Q.; Lai, X.J.; Su, X.J.; Liang, T.; Zeng, X.R. Thiolated Graphene-Based Superhydrophobic Sponges for Oil-Water Separation. Chem. Eng. J. 2017, 316, 736–743. [Google Scholar] [CrossRef]
- Wang, C.F.; Tzeng, F.S.; Chen, H.G.; Chang, C.J. Ultraviolet-Durable Superhydrophobic Zinc Oxide-Coated Mesh Films for Surface and Underwater-Oil Capture and Transportation. Langmuir 2012, 28, 10015–10019. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.L.; Jiang, H.B.; Ru, Y.; Zhang, X.H.; Qiao, J.L. Conductive Graphene-Melamine Sponge Prepared Via Microwave Irradiation. ACS Appl. Mater. Interfaces 2018, 10, 24776–24783. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.Q.; Pang, Y.Y.; Jiang, X.M.; Huang, J.; Xi, F.; Liu, J.Y. One-Step Fabrication of Novel Superhydrophobic and Superoleophilic Sponge with Outstanding Absorbency and Flame-Retardancy for the Selective Removal of Oily Organic Solvent from Water. Appl. Surf. Sci. 2018, 428, 338–347. [Google Scholar] [CrossRef]
- Lu, Y.Q.; Yuan, W.Z. Superhydrophobic/Superoleophilic and Reinforced Ethyl Cellulose Sponges for Oil/Water Separation: Synergistic Strategies of Cross-Linking, Carbon Nanotube Composite, and Nanosilica Modification. ACS Appl. Mater. Interfaces 2017, 9, 29167–29176. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, Y.Y.; Wang, Y.Q.; Yao, J.F. Furfuryl Alcohol Modified Melamine Sponge for Highly Efficient Oil Spill Clean-up and Recovery. J. Mater. Chem. A 2017, 5, 21893–21897. [Google Scholar] [CrossRef]
- Shi, J.; Alves, N.M.; Mano, J.F. Towards Bioinspired Superhydrophobic Poly(L-Lactic Acid) Surfaces Using Phase Inversion-Based Methods. Bioinspiration Biomim. 2008, 3, 034003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayer, I.S.; Steele, A.; Martorana, P.; Loth, E.; Robinson, S.J.; Stevenson, D. Biolubricant Induced Phase Inversion and Superhydrophobicity in Rubber-Toughened Biopolymer/Organoclay Nanocomposites. Appl. Phys. Lett. 2009, 95, 063702. [Google Scholar] [CrossRef]
- Vogelaar, L.; Lammertink, R.G.H.; Wessling, M. Superhydrophobic Surfaces Having Two-Fold Adjustable Roughness Prepared in a Single Step. Langmuir 2006, 22, 3125–3130. [Google Scholar] [CrossRef] [PubMed]
- Munirasu, S.; Banat, F.; Durrani, A.A.; Abu Haija, M. Intrinsically Superhydrophobic PVDF Membrane by Phase Inversion for Membrane Distillation. Desalination 2017, 417, 77–86. [Google Scholar] [CrossRef]
- Wang, C.F.; Lin, S.J. Robust Superhydrophobic/Superoleophilic Sponge for Effective Continuous Absorption and Expulsion of Oil Pollutants from Water. ACS Appl. Mater. Interfaces 2013, 5, 8861–8864. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, W.B.; Zhang, F.; Liu, X.; Wang, D.; Jin, J.; Jiang, L. Ultrafast Separation of Emulsified Oil/Water Mixtures by Ultrathin Free-Standing Single-Walled Carbon Nanotube Network Films. Adv. Mater. 2013, 25, 2422–2427. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.H.; Chen, D.Y.; Li, N.J.; Xu, Q.F.; Li, H.; He, J.H.; Lu, J.M. A Facile Method to Fabricate a Double-Layer Stainless Steel Mesh for Effective Separation of Water-in-Oil Emulsions with High Flux. J. Mater. Chem. A 2016, 4, 18815–18821. [Google Scholar] [CrossRef]
- Ge, D.T.; Yang, L.L.; Wang, C.B.; Lee, E.; Zhang, Y.Q.; Yang, S. A Multi-Functional Oil-Water Separator from a Selectively Pre-Wetted Superamphiphobic Paper. Chem. Commun. 2015, 51, 6149–6152. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, C.C.; Guo, C.Q.; Tian, H.F.; Zha, F.; Guo, L. Underoil Superhydrophilic Desert Sand Layer for Efficient Gravity-Directed Water-in-Oil Emulsions Separation with High Flux. J. Mater. Chem. A 2018, 6, 223–230. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.Z.; Wang, M.K.; Men, X.H.; Xue, Q.J. One-Pot Fabrication of Nanoporous Polymer Decorated Materials: From Oil-Collecting Devices to High-Efficiency Emulsion Separation. J. Mater. Chem. A 2017, 5, 5077–5087. [Google Scholar] [CrossRef]
- Ye, X.X.; Ke, L.; Wang, Y.P.; Gao, K.Y.; Cui, Y.W.; Wang, X.L.; Huang, X.; Shi, B. Polyphenolic-Chemistry-Enabled, Mechanically Robust, Flame Resistant and Superhydrophobic Membrane for Separation of Mixed Surfactant-Stabilized Emulsions. Chemistry 2018, 24, 10953–10958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Li, P.; Cao, B. Electrospun Microfibrous Membranes Based on Pim-1/Poss with High Oil Wettability for Separation of Oil-Water Mixtures and Cleanup of Oil Soluble Contaminants. Ind. Eng. Chem. Res. 2015, 54, 8772–8781. [Google Scholar] [CrossRef]
- Chen, L.W.; Si, Y.F.; Zhu, H.; Jiang, T.; Guo, Z.G. A Study on the Fabrication of Porous Pvdf Membranes by in-Situ Elimination and Their Applications in Separating Oil/Water Mixtures and Nano-Emulsions. J. Membr. Sci. 2016, 520, 760–768. [Google Scholar] [CrossRef]
- Zhang, W.B.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and Superoleophilic Pvdf Membranes for Effective Separation of Water-in-Oil Emulsions with High Flux. Adv. Mater. 2013, 25, 2071–2076. [Google Scholar] [CrossRef] [PubMed]








| Oil/water Mixtures | Oil Viscosity (mm2/s) | Flux (L m−2 h−1 bar−1) |
|---|---|---|
| isooctane/water | 0.654 | 12,740,000 |
| n-hexadecane/water | 3.526 | 3,640,000 |
| motor oil/water | 149.3 | 212,000 |
| viscous motor oil/water | 231.7 | 74,900 |
| Materials | The Flux of Surfactant Stabilized Water-in-Oil Emulsions | Oil Purity (wt %) | Ref. |
|---|---|---|---|
| poly-(N,Ndimethylaminoethyl methacrylate)/poly(divinylbenzene) modified stainless steel mesh | Up to 1200 L m−2 h−1 | >99.93 | [26] |
| fluorinated silica nanoparticles coated papers | >600 L m−2 h−1 | >99.9 | [27] |
| sand layer | Up to 2342 L m−2 h−1 | >99.98 | [28] |
| polydivinylbenzene/polydimethylsiloxane decorated filter membrane | none | >99.84 | [29] |
| superhydrophobic collagen fiber membrane | Up to 1627 L m−2 h−1 | >99.99 | [30] |
| PIM/polyhedral oligomeric silsesquioxane microfibrous membranes | Up to 1097 L m−2 h−1 | > 99.97 | [31] |
| porous PVDF membranes | Up to 318 L m−2 h−1 | > 99.64 | [32] |
| porous PVDF membranes | Up to 1000 L m−2 h−1 | > 99.95 | [33] |
| rough hydrophobic polymer coated stainless steel mesh | Up to 4235 L m−2 h−1 | > 99.99 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-F.; Tsai, Y.-J.; Kuo, S.-W.; Lee, K.-J.; Hu, C.-C.; Lai, J.-Y. Toward Superhydrophobic/Superoleophilic Materials for Separation of Oil/Water Mixtures and Water-in-Oil Emulsions Using Phase Inversion Methods. Coatings 2018, 8, 396. https://doi.org/10.3390/coatings8110396
Wang C-F, Tsai Y-J, Kuo S-W, Lee K-J, Hu C-C, Lai J-Y. Toward Superhydrophobic/Superoleophilic Materials for Separation of Oil/Water Mixtures and Water-in-Oil Emulsions Using Phase Inversion Methods. Coatings. 2018; 8(11):396. https://doi.org/10.3390/coatings8110396
Chicago/Turabian StyleWang, Chih-Feng, Yi-Jung Tsai, Shiao-Wei Kuo, Kuo-Jung Lee, Chien-Chieh Hu, and Juin-Yih Lai. 2018. "Toward Superhydrophobic/Superoleophilic Materials for Separation of Oil/Water Mixtures and Water-in-Oil Emulsions Using Phase Inversion Methods" Coatings 8, no. 11: 396. https://doi.org/10.3390/coatings8110396