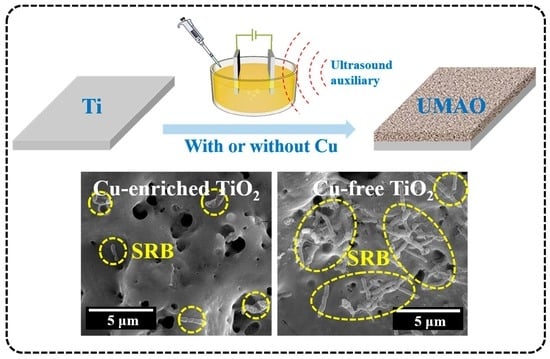
Ultrasound-Auxiliary Preparation of Antifouling Cu-Enriched Titanium Oxide Ceramic Layer
Abstract
:1. Introduction
2. Experimental Methods
2.1. Preparation of Specimens
2.2. Microstructural and Property Characterization
3. Results
4. Discussion
5. Conclusions
- Na2Cu-EDTA is a suitable Cu source for the fabrication of Cu-enriched TiO2 coating via the ultrasound-auxiliary micro-arc oxidation method.
- The addition of Na2Cu-EDTA results in a reduced rutile to anatase ratio in the resultant TiO2 coating.
- The Cu-enriched TiO2 coating is less defective relative to the Cu-free coating, which may thus exhibit enhanced corrosion resistance.
- The Cu enrichment of TiO2 benefits its antifouling capability against SRB.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schutz, R.W.; Watkins, H.B. Recent developments in titanium alloy application in the energy industry. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 1998, 243, 305–315. [Google Scholar] [CrossRef]
- Liu, Q.; Song, S.; Li, D.; Bai, Q. Research and Development of Titanium Alloy OCTG Application in Energy Industry. Oil Field Equip. 2014, 43, 88–94. [Google Scholar]
- Yao, J.; Wang, Y.; Wu, G.; Sun, M.; Wang, M.; Zhang, Q. Growth characteristics and properties of micro-arc oxidation coating on SLM-produced TC4 alloy for biomedical applications. Appl. Surf. Sci. 2019, 479, 727–737. [Google Scholar] [CrossRef]
- Barrino, F.; De La Rosa-Ramírez, H.; Schiraldi, C.; López-Martínez, J.; Samper, M.D. Preparation and Characterization of New Bioplastics Based on Polybutylene Succinate (PBS). Polymers 2023, 15, 1212. [Google Scholar] [CrossRef]
- Keasler, V.; De Paula, R.M.; Nilsen, G.; Grunwald, L.; Tidwell, T.J. 23—Biocides Overview and Applications in Petroleum Microbiology. In Trends in Oil and Gas Corrosion Research and Technologies; El-Sherik, A.M., Ed.; Woodhead Publishing: Boston, MA, USA, 2017; pp. 539–562. [Google Scholar]
- Venzlaff, H.; Enning, D.; Srinivasan, J.; Mayrhofer, K.J.J.; Hassel, A.W.; Widdel, F.; Stratmann, M. Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria. Corros. Sci. 2013, 66, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wen, F.; Cao, Y. Progress in Research of Corrosion and Protection by Sulfate-Reducing Bacteria. In Proceedings of the 3rd International Conference on Environmental Science And Information Application Technology Esiat 2011, Beijing, China, 18–19 June 2011; Volume 10, pp. 1177–1182. [Google Scholar]
- Qi, Y.; Liang, W.; Miao, Q.; Lin, H.; An, H.; Liu, Y.; Zuo, S.; Ma, H. Corrosion behavior and antifouling ability of Cu-Zn-Al/Zn-Al composite coating on Q235 steel. Surf. Coat. Technol. 2021, 405, 126614. [Google Scholar] [CrossRef]
- Tian, J.J.; Xu, K.W.; Hu, J.H.; Zhang, S.J.; Cao, G.Q.; Shao, G.S. Durable self-polishing antifouling Cu-Ti coating by a micron-scale Cu/Ti laminated microstructure design. J. Mater. Sci. Technol. 2021, 79, 62–74. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Zhou, S.; Wang, Y.; Wang, C.; Guo, W.; Lu, X.; Wang, L. Tailoring self-lubricating, wear-resistance, anticorrosion and antifouling properties of Ti/(Cu, MoS2)-DLC coating in marine environment by controlling the content of Cu dopant. Tribol. Int. 2020, 143, 106029. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Wang, J.; Xia, X.; Lv, Y.; Cai, G.; Liu, H.; Xiao, J.; Liu, B.; Dong, Z. Microstructure, formation mechanism and antifouling property of multi-layered Cu-incorporated Al2O3 coating fabricated through plasma electrolytic oxidation. Ceram. Int. 2019, 46, 2901–2909. [Google Scholar] [CrossRef]
- Yuan, J.P.; Li, W.; Wang, C. Effect of the La alloying addition on the antibacterial capability of 316L stainless steel. Mater. Sci. Eng. C 2013, 33, 446–452. [Google Scholar] [CrossRef]
- Li, Y.; Feng, S.; Liu, H.; Tian, X.; Xia, Y.; Li, M.; Xu, K.; Yu, H.; Liu, Q.; Chen, C. Bacterial distribution in SRB biofilm affects MIC pitting of carbon steel studied using FIB-SEM. Corros. Sci. 2020, 167, 108512. [Google Scholar] [CrossRef]
- Wang, P.; Lu, Z.; Zhang, D. Slippery liquid-infused porous surfaces fabricated on aluminum as a barrier to corrosion induced by sulfate reducing bacteria. Corros. Sci. 2015, 93, 159–166. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, J.; Sun, C.; Yu, Z.; Hou, B. The corrosion of two aluminium sacrificial anode alloys in SRB-containing sea mud. Corros. Sci. 2014, 83, 375–381. [Google Scholar] [CrossRef]
- Gu, T.; Jia, R.; Unsal, T.; Xu, D. Toward a better understanding of microbiologically influenced corrosion caused by sulfate reducing bacteria. J. Mater. Sci. Technol. 2019, 35, 631–636. [Google Scholar] [CrossRef]
- Rao, T.S.; Kora, A.J.; Anupkumar, B.; Narasimhan, S.V.; Feser, R. Pitting corrosion of titanium by a freshwater strain of sulphate reducing bacteria (Desulfovibrio vulgaris). Corros. Sci. 2005, 47, 1071–1084. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, Z.; Lu, X.; Lv, Y.; Cai, G.; Yang, L.; Dong, Z. Microstructural evolution and biological performance of Cu-incorporated TiO2 coating fabricated through one-step micro-arc oxidation. Appl. Surf. Sci. 2019, 508, 144766. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Lv, Y.; Zhang, X.; Dong, Z.; Yang, L.; Zhang, E. Ag distribution and corrosion behaviour of the plasma electrolytic oxidized antibacterial Mg-Ag alloy. Electrochim. Acta 2022, 411, 140089. [Google Scholar] [CrossRef]
- Li, K.; Xia, C.; Qiao, Y.; Liu, X. Dose-response relationships between copper and its biocompatibility/antibacterial activities. J. Trace Elem. Med. Biol. 2019, 55, 127–135. [Google Scholar] [CrossRef]
- Cerchier, P.; Pezzato, L.; Moschin, E.; Coelho, L.B.; Olivier, M.G.M.; Moro, I.; Magrini, M. Antifouling properties of different Plasma Electrolytic Oxidation coatings on 7075 aluminium alloy. Int. Biodeterior. Biodegrad. 2018, 133, 70–78. [Google Scholar] [CrossRef]
- Wang, J.; Yang, C.; Liu, S.; Fang, S.; Xia, C. Novel Antifouling Technology Research: Progress and Prospects. Sci. Sin. Vitae 2016, 46, 1079–1084. [Google Scholar]
- Qian, P.Y.; Xu, Y.; Fusetani, N. Natural products as antifouling compounds: Recent progress and future perspectives. Biofouling 2010, 26, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yan, Z.; Cao, L. Bake hardening behavior and precipitation kinetic of a novel Al-Mg-Si-Cu aluminum alloy for lightweight automotive body. Mater. Sci. Eng. A 2018, 728, 88–94. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, T.; Lv, Y.; Zhang, Y.; Lu, X.; Xiao, J.; Ma, C.; Li, Z.; Dong, Z. Enhanced uniformity, corrosion resistance and biological performance of Cu-incorporated TiO2 coating produced by ultrasound-auxiliary micro-arc oxidation. Appl. Surf. Sci. 2021, 569, 150932. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Jiang, D.; Jiao, Y.; Wu, Y.; Peng, Z.; Zhou, J.; Wu, J.; Dong, Z. Synthesis and characterization of a bi-functional hydroxyapatite/Cu-doped TiO2 composite coating. Ceram. Int. 2019, 45, 6693–6701. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Wang, X.; Wang, Y.; Hang, R.; Huang, X.; Tang, B.; Chu, P.K. Effects of copper nanoparticles in porous TiO2 coatings on bacterial resistance and cytocompatibility of osteoblasts and endothelial cells. Mater. Sci. Eng. C 2018, 82, 110–120. [Google Scholar] [CrossRef]
- He, X.; Zhang, G.; Wang, X.; Hang, R.; Huang, X.; Qin, L.; Tang, B.; Zhang, X. Biocompatibility, corrosion resistance and antibacterial activity of TiO2/CuO coating on titanium. Ceram. Int. 2017, 43, 16185–16195. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, L.; Hou, Y.; Duan, G.; Yu, B.; Li, X.; Zhai, Y.; Zhang, B.; Zhang, T.; Wang, F. Grain refinement promotes the formation of phosphate conversion coating on Mg alloy AZ91D with high corrosion resistance and low electrical contact resistance. Corros. Commun. 2021, 1, 47–57. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, X.R.; Curioni, M.; Pawar, S.; Liu, H.; Fan, Z.Y.; Scamans, G.; Thompson, G. Corrosion Behavior of Pure Magnesium with Low Iron Content in 3.5 wt% NaCl Solution. J. Electrochem. Soc. 2015, 162, C362–C368. [Google Scholar] [CrossRef]
- Buchheit, R.G. A Compilation of Corrosion Potentials Reported for Intermetallic Phases in Aluminum Alloys. J. Electrochem. Soc. 1995, 142, 3994–3996. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Z.; Wang, Y.; Li, J. Tribological mechanism of micro-arc oxidation coatings prepared by different electrolyte systems in artificial seawater. Ceram. Int. 2021, 47, 7344–7352. [Google Scholar] [CrossRef]
- Oliveira, F.G.; Ribeiro, A.R.; Perez, G.; Archanjo, B.S.; Gouvea, C.P.; Araújo, J.R.; Campos, A.P.C.; Kuznetsov, A.; Almeida, C.M.; Maru, M.M.; et al. Understanding growth mechanisms and tribocorrosion behaviour of porous TiO2 anodic films containing calcium, phosphorous and magnesium. Appl. Surf. Sci. 2015, 341, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Rokosz, K.; Hryniewicz, T.; Matysek, D.; Raaen, S.; Valicek, J.; Dudek, L.; Harnicarova, M. SEM, EDS and XPS Analysis of the Coatings Obtained on Titanium after Plasma Electrolytic Oxidation in Electrolytes Containing Copper Nitrate. Materials 2016, 9, 318. [Google Scholar] [CrossRef]
- Yoshimura, R.; Konno, T.J.; Abe, E.; Hiraga, K. Transmission electron microscopy study of the evolution of precipitates in aged Al–Li–Cu alloys: The θ′ and T1 phases. Acta Mater. 2003, 51, 4251–4266. [Google Scholar] [CrossRef]
- Dejiu, S.; Jingrui, C.; Guolong, L.; Donglei, H.; Lailei, W.; Haojie, M.; Yonghong, X.; He, C.; Yaqian, Y. Effect of ultrasonic on microstructure and growth characteristics of micro-arc oxidation ceramic coatings on 6061 aluminum alloy. Vacuum 2014, 99, 143–148. [Google Scholar] [CrossRef]
- Qu, L.; Li, M.; Liu, M.; Zhang, E.; Ma, C. Microstructure and corrosion resistance of ultrasonic micro-arc oxidation biocoatings on magnesium alloy. J. Adv. Ceram. 2013, 2, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Lv, Y.; Sun, S.; Zhang, X.; Lu, X.; Dong, Z. Construction of multi-layered Zn-modified TiO2 coating by ultrasound-auxiliary micro-arc oxidation: Microstructure and biological property. Mater. Sci. Eng. C 2021, 131, 112487. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhang, C.; Zhang, Y.; Wang, Q.; Zhang, X.; Dong, Z. Microstructure and Corrosion Resistance of Plasma Electrolytic Oxidized Recycled Mg Alloy. Acta Metall. Sin. Engl. Lett. 2022, 35, 961–974. [Google Scholar] [CrossRef]
- Wang, S.; Deng, L.; Lv, Y.; Zhang, T.; Zhang, X.; Dong, Z.; Cai, G. Construction of antifouling Cu-modified TiO2 coating via micro-arc oxidation: The influence of Cu content. Surf. Coat. Technol. 2023, 454, 129197. [Google Scholar] [CrossRef]
- Permeh, S.; Lau, K.; Duncan, M. Effect of crevice morphology on SRB activity and steel corrosion under marine foulers. Bioelectrochemistry 2021, 142, 107922. [Google Scholar] [CrossRef]
- Li, M.; Seyeux, A.; Wiame, F.; Marcus, P.; Światowska, J. Insights on the Al-Cu-Fe-Mn intermetallic particles induced pitting corrosion of Al-Cu-Li alloy. Corros. Sci. 2020, 176, 109040. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. An investigation of ceramic coating growth mechanisms in plasma electrolytic oxidation (PEO) processing. Electrochim. Acta 2013, 112, 111–119. [Google Scholar] [CrossRef]
- Zhu, L.; Guo, Z.; Zhang, Y.; Li, Z.; Sui, M. A mechanism for the growth of a plasma electrolytic oxide coating on Al. Electrochim. Acta 2016, 208, 296–303. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Kamil, M.P.; Kaseem, M.; Ko, Y.G. Soft plasma electrolysis with complex ions for optimizing electrochemical performance. Sci. Rep. 2017, 7, 44458. [Google Scholar] [CrossRef] [Green Version]
- Li, C.Y.; Fan, X.L.; Zeng, R.C.; Cui, L.Y.; Li, S.Q.; Zhang, F.; He, Q.K.; Kannan, M.B.; Jiang, H.W.; Chen, D.C.; et al. Corrosion resistance of in-situ growth of nano-sized Mg(OH)2 on micro-arc oxidized magnesium alloy AZ31-Influence of EDTA. J. Mater. Sci. Technol. 2019, 35, 1088–1098. [Google Scholar] [CrossRef]
- Lu, X.; Mohedano, M.; Blawert, C.; Matykina, E.; Arrabal, R.; Kainer, K.U.; Zheludkevich, M.L. Plasma electrolytic oxidation coatings with particle additions—A review. Surf. Coat. Technol. 2016, 307, 1165–1182. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Yu, Y.; Lu, X.; Lv, Y.; Jiang, D.; Peng, Z.; Zhou, J.; Zhang, X.; Sun, S.; et al. Characterization and property of bifunctional Zn-incorporated TiO2 micro-arc oxidation coatings: The influence of different Zn sources. Ceram. Int. 2019, 45, 19747–19756. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Lu, X.; Lv, Y.; Jiang, D.; Yu, Y.; Peng, Z.; Dong, Z. Characterization and property of dual-functional Zn-incorporated TiO2 micro-arc oxidation coatings: The influence of current density. J. Alloys Compd. 2019, 810, 151893. [Google Scholar] [CrossRef]
- Treacy, G.M.; Rudd, A.L.; Breslin, C.B. Electrochemical behaviour of aluminium in the presence of EDTA-containing chloride solutions. J. Appl. Electrochem. 2000, 30, 675–683. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Cao, G.; Gu, T.; Wang, C.; Wang, Z.; Sun, C. The corrosion behavior of the 6061 Al alloy in simulated Nansha marine atmosphere. J. Mater. Res. Technol. 2022, 19, 709–721. [Google Scholar] [CrossRef]
- Tweddle, D.; Johnson, J.A.; Kapoor, M.; Bikmukhametov, I.; Mileski, S.; Carsley, J.E.; Thompson, G.B. Atomic-scale Clustering in a High-strength Al-Mg-Si-Cu Alloy. Materialia 2022, 26, 101567. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.; Liu, C.; Wang, H.; Ren, B.; Yang, K.; Zhang, E. Effect of Cu content on the antibacterial activity of titanium–copper sintered alloys. Mater. Sci. Eng. C 2014, 35, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Jo, Y.H.; Kayani, S.H.; Kim, H.-W.; Lee, J.-H. Effects of Cu additions on the precipitation activation energy and mechanical properties of prestrained Al–Mg–Si alloys. J. Mater. Res. Technol. 2022, 20, 2629–2637. [Google Scholar] [CrossRef]










| Element | Content (wt.%) | ||||
|---|---|---|---|---|---|
| Ti | O | Ca | P | Cu | |
| Cu-enriched TiO2 | 52.46 | 34.00 | 5.05 | 5.95 | 2.54 |
| Cu-free TiO2 | 55.04 | 34.60 | 5.14 | 5.20 | --- |
| Samples | βa (mV) | βc (mV) | icorr (A/cm2) | Ecorr (V) |
|---|---|---|---|---|
| Bare Ti | 177.65 | 133.83 | 7.933 × 10−7 | −0.26 |
| Cu-free TiO2 | 166.88 | 56.73 | 1.509 × 10−8 | 0.084 |
| Cu-enriched TiO2 | 363.67 | 49.60 | 4.957 × 10−9 | 0.203 |
| Samples | Rs (Ω·cm2) | CPEcoat-Q (F/cm2) | CPEcoat-n | Rcoat (Ω·cm2) | CPEdl-Q (F/cm2) | CPEdl-n | Rct (Ω·cm2) |
|---|---|---|---|---|---|---|---|
| Bare Ti | 5.61 | 2.726 × 10−5 | 0.850 | 8436 | 2.719 × 10−4 | 0.647 | 4.212 × 104 |
| Cu-free TiO2 | 8.61 | 5.193 × 10−4 | 0.702 | 72.2 | 3.546 × 10−5 | 0.723 | 2.085 × 105 |
| Cu-enriched TiO2 | 7.02 | 4.084 × 10−4 | 0.727 | 67.2 | 2.743 × 10−5 | 0.747 | 2.486 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Cheng, Y.; Meng, X.; Jiao, X.; Dong, Z.; Zhang, X. Ultrasound-Auxiliary Preparation of Antifouling Cu-Enriched Titanium Oxide Ceramic Layer. Coatings 2023, 13, 1099. https://doi.org/10.3390/coatings13061099
Lv Y, Cheng Y, Meng X, Jiao X, Dong Z, Zhang X. Ultrasound-Auxiliary Preparation of Antifouling Cu-Enriched Titanium Oxide Ceramic Layer. Coatings. 2023; 13(6):1099. https://doi.org/10.3390/coatings13061099
Chicago/Turabian StyleLv, You, Yubing Cheng, Xiangzhe Meng, Xu Jiao, Zehua Dong, and Xinxin Zhang. 2023. "Ultrasound-Auxiliary Preparation of Antifouling Cu-Enriched Titanium Oxide Ceramic Layer" Coatings 13, no. 6: 1099. https://doi.org/10.3390/coatings13061099