Photoluminescent Coatings on Zinc Alloy Prepared by Plasma Electrolytic Oxidation in Aluminate Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Setup for PEO Anodization
2.3. Characterization Techniques
3. Results and Discussion
3.1. Electrochemical Behavior of Zinc Alloy Electrodes at Anodic Polarization in Alkaline Aluminate-Containing Electrolyte
3.2. PEO Coating Morphology and Elemental Composition
3.3. Crystalline Structure of the PEO Coatings
3.4. Diffuse Reflectance Spectra of the PEO Coatings
3.5. Spectral Characterization of Microdischarge Emission during PEO Process on Zn Electrodes in Aluminate-Based Electrolyte
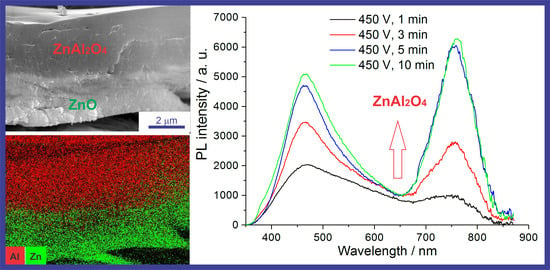
3.6. Photoluminescence Properties of the PEO Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Adhesion/Cohesion Behavior
Appendix A.2. Wear Behavior





Appendix A.3. The Synthesis of ZnAl2O4 Powder

References
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma Electrolytic Oxidation (Peo) Process—Processing, Properties, and Applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent Progress in Surface Modification of Metals Coated by Plasma Electrolytic Oxidation: Principle, Structure, and Performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma Electrolysis for Surface Engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Wood, G.C.; Pearson, C. Dielectric Breakdown of Anodic Oxide Films on Valve Metals. Corros. Sci. 1967, 7, 119–125. [Google Scholar] [CrossRef]
- Yahalom, J.; Hoar, T.P. Galvanostatic Anodizing of Aluminium. Electrochim. Acta 1970, 15, 877–884. [Google Scholar] [CrossRef]
- Dyer, C.K.; Leach, J.S.L. Breakdown and Efficiency of Anodic Oxide Growth on Titanium. J. Electrochem. Soc. 1978, 125, 1032–1038. [Google Scholar] [CrossRef]
- Kadary, V.; Klein, N. Electrical Breakdown: I. During the Anodic Growth of Tantalum Pentoxide. J. Electrochem. Soc. 1980, 127, 139–151. [Google Scholar] [CrossRef]
- Bakovets, V.V.; Polyakov, O.V.; Dolgovesova, I.P. Plasma Electrolytic Anodic Treatment of Metals; Nauka: Novosibirsk, Russia, 1991. [Google Scholar]
- Chernenko, V.I.; Snezhko, L.A.; Papanova, I.I. Coating Preparation by Anodic Spark Electrolysis; Khimiya: Leningrad, Russia, 1991. [Google Scholar]
- Voevodin, A.A.; Yerokhin, A.L.; Lyubimov, V.V.; Donley, M.S.; Zabinski, J.S. Characterization of Wear Protective Al-Si-O Coatings Formed on Al-Based Alloys by Micro-Arc Discharge Treatment. Surf. Coat. Technol. 1996, 86–87, 516–521. [Google Scholar] [CrossRef]
- Egorkin, V.S.; Gnedenkov, S.V.; Sinebryukhov, S.L.; Vyaliy, I.E.; Gnedenkov, A.S.; Chizhikov, R.G. Increasing Thickness and Protective Properties of PEO-Coatings on Aluminum Alloy. Surf. Coat. Technol. 2018, 334, 29–42. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Pardo, A.; Mohedano, M.; Mingo, B.; Rodríguez, I.; González, J. Energy-Efficient PEO Process of Aluminium Alloys. Mater. Lett. 2014, 127, 13–16. [Google Scholar] [CrossRef] [Green Version]
- Sieber, M.; Simchen, F.; Morgenstern, R.; Scharf, I.; Lampke, T. Plasma Electrolytic Oxidation of High-Strength Aluminium Alloys—Substrate Effect on Wear and Corrosion Performance. Metals 2018, 8, 365. [Google Scholar] [CrossRef] [Green Version]
- Yerokhin, A.L.; Leyland, A.; Matthews, A. Kinetic Aspects of Aluminium Titanate Layer Formation on Titanium Alloys by Plasma Electrolytic Oxidation. Appl. Surf. Sci. 2002, 200, 172–184. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Molaei, M.; Babaei, K. Influence of Electrolyte Composition and Voltage on the Microstructure and Growth Mechanism of Plasma Electrolytic Oxidation (PEO) Coatings on Tantalum: A Review. Anal. Bioanal. Electrochem. 2020, 12, 517–535. [Google Scholar] [CrossRef]
- Quintero, D.; Gómez, M.A.; Araujo, W.S.; Echeverría, F.; Calderón, J.A. Influence of the Electrical Parameters of the Anodizing PEO Process on Wear and Corrosion Resistance of Niobium. Surf. Coat. Technol. 2019, 380, 125067. [Google Scholar] [CrossRef]
- Cengiz, S.; Uzunoglu, A.; Stanciu, L.; Tarakci, M.; Gencer, Y. Direct Fabrication of Crystalline Hydroxyapatite Coating on Zirconium by Single-Step Plasma Electrolytic Oxidation Process. Surf. Coat. Technol. 2016, 301, 74–79. [Google Scholar] [CrossRef]
- Echeverry-Rendon, M.; Duque, V.; Quintero, D.; Harmsen, M.C.; Echeverria, F. Novel Coatings Obtained by Plasma Electrolytic Oxidation to Improve the Corrosion Resistance of Magnesium-Based Biodegradable Implants. Surf. Coat. Technol. 2018, 354, 28–37. [Google Scholar] [CrossRef]
- Stojadinović, S.; Tadić, N.; Vasilić, R. Formation and Characterization of ZnO Films on Zinc Substrate by Plasma Electrolytic Oxidation. Surf. Coat. Technol. 2016, 307, 650–657. [Google Scholar] [CrossRef]
- Rocca, E.; Veys-Renaux, D.; Guessoum, K. Electrochemical Behavior of Zinc in KOH Media at High Voltage: Micro-Arc Oxidation of Zinc. J. Electroanal. Chem. 2015, 754, 125–132. [Google Scholar] [CrossRef]
- Malinovschi, V.; Marin, A.; Mihalache, M.; Iosub, I. Preparation and Characterization of Coatings on Carbon Steel Obtained by PEO in Silicate/Carbonate Electrolyte. Surf. Coat. Technol. 2016, 296, 96–103. [Google Scholar] [CrossRef]
- Karpushenkov, S.A.; Shchukin, G.L.; Belanovich, A.L.; Savenko, V.P.; Kulak, A.I. Plasma Electrolytic Ceramic-like Aluminum Oxide Coatings on Iron. J. Appl. Electrochem. 2010, 40, 365–374. [Google Scholar] [CrossRef]
- Yang, W.; Peng, Z.; Liu, B.; Liu, W.; Liang, J. Influence of Silicate Concentration in Electrolyte on the Growth and Performance of Plasma Electrolytic Oxidation Coatings Prepared on Low Carbon Steel. J. Mater. Eng. Perform. 2018, 27, 2345–2353. [Google Scholar] [CrossRef]
- Serdechnova, M.; Blawert, C.; Karpushenkov, S.; Karpushenkava, L.; Shulha, T.; Karlova, P.; Vasilić, R.; Stojadinović, S.; Stojanović, S.; Damjanović-Vasilić, L.; et al. Properties of ZnO/ZnAl2O4 Composite PEO Coatings on Zinc Alloy Z1. Surf. Coat. Technol. 2021, 410, 126948. [Google Scholar] [CrossRef]
- Miron, I.; Enache, C.; Vasile, M.; Grozescu, I. Optical Properties of ZnAl2O4 Nanomaterials Obtained by the Hydrothermal Method. Phys. Scr. 2012, T149, 014064. [Google Scholar] [CrossRef]
- Ghribi, F.; Sehailia, M.; Aoudjit, L.; Touahra, F.; Zioui, D.; Boumechhour, A.; Halliche, D.; Bachari, K.; Benmaamar, Z. Solar-Light Promoted Photodegradation of Metronidazole over ZnO-ZnAl2O4 Heterojunction Derived from 2D-Layered Double Hydroxide Structure. J. Photochem. Photobiol. A Chem. 2020, 397, 112510. [Google Scholar] [CrossRef]
- Menon, S.; Dhabekar, B.; Alagu Raja, E.; More, S.P.; Gundu Rao, T.K.; Kher, R.K. TSL, OSL and ESR Studies in ZnAl2O4:Tb Phosphor. J. Lumin. 2008, 128, 1673–1678. [Google Scholar] [CrossRef]
- Sampath, S.K.; Cordaro, J.F. Optical Properties of Zinc Aluminate, Zinc Gallate, and Zinc Aluminogallate Spinels. J. Am. Ceram. Soc. 1998, 81, 649–654. [Google Scholar] [CrossRef]
- Lou, Z.; Hao, J. Cathodoluminescence of Rare-Earth-Doped Zinc Aluminate Films. Thin Solid Films 2004, 450, 334–340. [Google Scholar] [CrossRef]
- Farhadi, S.; Panahandehjoo, S. Spinel-Type Zinc Aluminate (ZnAl2O4) Nanoparticles Prepared by the Co-Precipitation Method: A Novel, Green and Recyclable Heterogeneous Catalyst for the Acetylation of Amines, Alcohols and Phenols under Solvent-Free Conditions. Appl. Catal. A Gen. 2010, 382, 293–302. [Google Scholar] [CrossRef]
- Matveyeva, A.N.; Omarov, S.O.; Nashchekin, A.V.; Popkov, V.I.; Murzin, D.Y. Catalyst support based on ZnO-ZnAl2O4 nanocomposites with enhanced selectivity and coking resistance in isobutane dehudrogenation. Dalton Trans. 2022, 51, 12213. [Google Scholar] [CrossRef]
- Liu, L.; Lin, Y.; Hu, Y.; Lin, Z.; Lin, S.; Du, M.; Zhang, L.; Zhang, X.; Lin, J.; Zhang, Z.; et al. ZnAl2O4 Spinel-Supported PdZnβ Catalyst with Parts per Million Pd for Methanol Steam Reforming. ACS Catal. 2022, 12, 2714–2721. [Google Scholar] [CrossRef]
- Song, L.; Wang, H.; Wang, S.; Qu, Z. Dual-site activation of H2 over Cu/ZnAl2O4 boosting CO2 hydrogenation to methanol. Appl. Catal. B. 2023, 322, 122137. [Google Scholar] [CrossRef]
- Moustafa, M.G.; Sanad, M.M.S. Green fabrication of ZnAl2O4-coated LiFePO4 nanoparticles for enhanced electrochemical performance in Li-ion batteries. J. Alloy. Compd. 2022, 903, 163910. [Google Scholar] [CrossRef]
- Rojas-Hernandez, R.E.; Rubio-Marcos, F.; Romet, I.; del Campo, A.; Gorni, G.; Hussainova, I.; Fernandez, J.F.; Nagirnyi, V. Deep-Ultraviolet Emitter: Rare-Earth-Free ZnAl2O4 Nanofibers via a Simple Wet Chemical Route. Inorg. Chem. 2022, 61, 11886–11896. [Google Scholar] [CrossRef]
- Gouadria, S.; Abudllah, M.; Ahmad, Z.; John, P.; Nisa, M.U.; Manzoor, S.; Aman, S.; Ashiq, M.N.; Ghori, M.I. Development of bifunctional Mo doped ZnAl2O4 spinel nanorods array directly grown on carbon fiber for supercapacitor and OER application. Ceram. Int. 2023, 49, 4281–4289. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Shailajha, S.; Seema, S.; Mubina, M.S.K. Enhanced Magneto-Electric Properties of ZnAl2O4@NiFe2O4 Nanocomposites in Magnetic Sensor Applications. J. Supercond. Nov. Magn. 2023, 36, 693–709. [Google Scholar] [CrossRef]
- Maltanava, H.; Poznyak, S.; Ovodok, E.; Ivanovskaya, M.; Maia, F.; Kudlash, A.; Scharnagl, N.; Tedim, J. Synthesis of ZnO Mesoporous Powders and Their Application in Dye Photodegradation. Mater. Today Proc. 2018, 5, 17414–17421. [Google Scholar] [CrossRef]
- Byeon, K.J.; Cho, J.Y.; Jo, H.B.; Lee, H. Fabrication of High-Brightness GaN-Based Light-Emitting Diodes via Thermal Nanoimprinting of ZnO-Nanoparticle-Dispersed Resin. Appl. Surf. Sci. 2015, 346, 354–360. [Google Scholar] [CrossRef]
- Battiston, S.; Rigo, C.; Da Cruz Severo, E.; Mazutti, M.A.; Kuhn, R.C.; Gündel, A.; Foletto, E.L. Synthesis of Zinc Aluminate (ZnAl2O4) Spinel and Its Application as Photocatalyst. Mater. Res. 2014, 17, 734–738. [Google Scholar] [CrossRef]
- Nasr, M.; Viter, R.; Eid, C.; Warmont, F.; Habchi, R.; Miele, P.; Bechelany, M. Synthesis of Novel ZnO/ZnAl2O4 Multi Co-Centric Nanotubes and Their Long-Term Stability in Photocatalytic Application. RSC Adv. 2016, 6, 103692–103699. [Google Scholar] [CrossRef]
- Moon, S.; Jeong, Y. Generation Mechanism of Microdischarges during Plasma Electrolytic Oxidation of Al in Aqueous Solutions. Corros. Sci. 2009, 51, 1506–1512. [Google Scholar] [CrossRef]
- Guo, H.F.; An, M.Z. Growth of Ceramic Coatings on AZ91D Magnesium Alloys by Micro-Arc Oxidation in Aluminate-Fluoride Solutions and Evaluation of Corrosion Resistance. Appl. Surf. Sci. 2005, 246, 229–238. [Google Scholar] [CrossRef]
- Mécuson, F.; Czerwiec, T.; Belmonte, T.; Dujardin, L.; Viola, A.; Henrion, G. Diagnostics of an Electrolytic Microarc Process for Aluminium Alloy Oxidation. Surf. Coat. Technol. 2005, 200, 804–808. [Google Scholar] [CrossRef]
- Prieditis, G.; Feldbach, E.; Kudryavtseva, I.; Popov, A.I.; Shablonin, E.; Lushchik, A. Luminescence Characteristics of Magnesium Aluminate Spinel Crystals of Different Stoichiometry. In IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2019; Volume 503, p. 012021. [Google Scholar] [CrossRef]
- Sun, X.; Jiang, Z.; Yao, Z.; Zhang, X. The Effects of Anodic and Cathodic Processes on the Characteristics of Ceramic Coatings Formed on Titanium Alloy through the MAO Coating Technology. Appl. Surf. Sci. 2005, 252, 441–447. [Google Scholar] [CrossRef]
- Kubelka, P. New Contributions to the Optics of Intensely Light-Scattering Materials. J. Opt. Soc. Am. 1948, 38, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Brik, M.G.; Srivastava, A.M.; Popov, A.I. A Few Common Misconceptions in the Interpretation of Experimental Spectroscopic Data. Opt. Mater. 2022, 127, 112276. [Google Scholar] [CrossRef]
- Srikant, V.; Clarke, D.R. On the Optical Band Gap of Zinc Oxide. J. Appl. Phys. 1998, 83, 5447–5451. [Google Scholar] [CrossRef]
- Arbi, M.; Benramdane, N.; Kebbab, Z.; Miloua, R.; Chiker, F.; Khenata, R. First Principles Calculations of Structural, Electronic and Optical Properties of Zinc Aluminum Oxide. Mater. Sci. Semicond. Process. 2012, 15, 301–307. [Google Scholar] [CrossRef]
- Rafla-Yuan, H.; Cordaro, J.F. Optical Reflectance of Aluminum-Doped Zinc Oxide Powders. J. Appl. Phys. 1991, 69, 959–964. [Google Scholar] [CrossRef]
- Ragupathi, C.; John Kennedy, L.; Judith Vijaya, J. A New Approach: Synthesis, Characterization and Optical Studies of Nano-Zinc Aluminate. Adv. Powder Technol. 2014, 25, 267–273. [Google Scholar] [CrossRef]
- Sampath, S.K.; Kanhere, D.G.; Pandey, R. Electronic Structure of Spinel Oxides: Zinc Aluminate and Zinc Gallate. J. Phys. Condens. Matter 1999, 11, 3635–3644. [Google Scholar] [CrossRef] [Green Version]
- Mathur, S.; Veith, M.; Haas, M.; Shen, H.; Lecerf, N.; Huch, V.; Hüfner, S.; Haberkorn, R.; Beck, H.P.; Jilavi, M. Single-Source Sol-Gel Synthesis of Nanocrystalline ZnAl2O4: Structural and Optical Properties. J. Am. Ceram. Soc. 2001, 84, 1921–1928. [Google Scholar] [CrossRef]
- Stojadinović, S.; Jovović, J.; Petković, M.; Vasilić, R.; Konjević, N. Spectroscopic and Real-Time Imaging Investigation of Tantalum Plasma Electrolytic Oxidation (PEO). Surf. Coat. Technol. 2011, 205, 5406–5413. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O.; Yerokhin, A.; Matthews, A. Spectroscopic Study of Electrolytic Plasma and Discharging Behaviour during the Plasma Electrolytic Oxidation (PEO) Process. J. Phys. D Appl. Phys. 2010, 43, 105203. [Google Scholar] [CrossRef]
- Dunleavy, C.S.; Golosnoy, I.O.; Curran, J.A.; Clyne, T.W. Characterisation of Discharge Events during Plasma Electrolytic Oxidation. Surf. Coat. Technol. 2009, 203, 3410–3419. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A Review on ZnO: Fundamental Properties and Applications. Mater. Today Proc. 2022, 49, 3028–3035. [Google Scholar] [CrossRef]
- Ayoub, I.; Kumar, V.; Abolhassani, R.; Sehgal, R.; Sharma, V.; Sehgal, R.; Swart, H.C.; Mishra, Y.K. Advances in ZnO: Manipulation of Defects for Enhancing Their Technological Potentials. Nanotechnol. Rev. 2022, 11, 575–619. [Google Scholar] [CrossRef]
- Du, B.; Zhang, M.; Ye, J.; Wang, D.; Han, J.; Zhang, T. Novel Au Nanoparticle-Modified ZnO Nanorod Arrays for Enhanced Photoluminescence-Based Optical Sensing of Oxygen. Sensors 2023, 23, 2886. [Google Scholar] [CrossRef]
- Jiang, T.T.; Du, B.S.; Zhang, H.; Yu, D.F.; Sun, L.; Zhao, G.Y.; Yang, C.H.; Sun, Y.; Yu, M.; Ashfold, M.N.R. High-performance photoluminescence-based oxygen sensing with Pr-modified ZnO nanofibers. Appl. Surf. Sci. 2019, 483, 922–928. [Google Scholar] [CrossRef] [Green Version]
- da Silva, A.A.; Gonçalves, A.S.; Davolos, M.R.; Santagneli, S.H. Al3+ environments in nanostructured ZnAl2O4 and their effects on the luminescence properties. J. Nanosci. Nanotechnol. 2008, 8, 5690–5695. [Google Scholar] [CrossRef]
- Zeng, H.; Duan, G.; Li, Y.; Yang, S.; Xu, X.; Cai, W. Blue Luminescence of ZnO Nanoparticles Based on Non-Equilibrium Processes: Defect Origins and Emission Controls. Adv. Funct. Mater. 2010, 20, 561–572. [Google Scholar] [CrossRef]
- Vanheusden, K.; Warren, W.L.; Seager, C.H.; Tallant, D.R.; Voigt, J.A.; Gnade, B.E. Mechanisms behind Green Photoluminescence in ZnO Phosphor Powders. J. Appl. Phys. 1996, 79, 7983–7990. [Google Scholar] [CrossRef]
- Greene, L.E.; Law, M.; Goldberger, J.; Kim, F.; Johnson, J.C.; Zhang, Y.; Saykally, R.J.; Yang, P. Low-Temperature Wafer-Scale Production of ZnO Nanowire Arrays. Angew. Chem.—Int. Ed. 2003, 42, 3031–3034. [Google Scholar] [CrossRef] [PubMed]
- Van Dijken, A.; Meulenkamp, E.A.; Vanmaekelbergh, D.; Meijerink, A. Luminescence of Nanocrystalline ZnO Particles: The Mechanism of the Ultraviolet and Visible Emission. J. Lumin. 2000, 87, 454–456. [Google Scholar] [CrossRef]
- Studenikin, S.A.; Golego, N.; Cocivera, M. Fabrication of Green and Orange Photoluminescent, Undoped ZnO Films Using Spray Pyrolysis. J. Appl. Phys. 1998, 84, 2287–2294. [Google Scholar] [CrossRef]
- Sun, G.; Sun, G.; Zhong, M.; Wang, S.; Zu, X.; Xiang, X. Coordination Mechanism, Characterization, and Photoluminescence Properties of Spinel ZnAl2O4 Nanoparticles Prepared by a Modified Polyacrylamide Gel Route. Russ. J. Phys. Chem. A 2016, 90, 691–699. [Google Scholar] [CrossRef]


















| Time of the Treatment, min | Applied Voltage: 370 V | Applied Voltage: 450 V | ||||
|---|---|---|---|---|---|---|
| O | Al | Zn | O | Al | Zn | |
| 1 | 59.62 | 26.30 | 14.09 | 60.66 | 26.40 | 12.93 |
| 3 | 60.72 | 27.62 | 11.61 | 60.97 | 31.05 | 7.98 |
| 5 | 60.71 | 27.13 | 12.16 | 62.90 | 30.61 | 6.49 |
| 10 | 59.74 | 24.09 | 16.17 | 59.93 | 33.04 | 7.03 |
| Time of the PEO Treatment, min | Applied Voltage, V | |
|---|---|---|
| 370 | 450 | |
| 1 | 3.38 eV | 3.29 eV |
| 3 | 3.33 eV | 3.29 eV |
| 5 | 3.32 eV | 3.27 eV |
| 10 | 3.28 eV | 3.26 eV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maltanava, H.; Stojadinovic, S.; Vasilic, R.; Karpushenkov, S.; Belko, N.; Samtsov, M.; Poznyak, S. Photoluminescent Coatings on Zinc Alloy Prepared by Plasma Electrolytic Oxidation in Aluminate Electrolyte. Coatings 2023, 13, 848. https://doi.org/10.3390/coatings13050848
Maltanava H, Stojadinovic S, Vasilic R, Karpushenkov S, Belko N, Samtsov M, Poznyak S. Photoluminescent Coatings on Zinc Alloy Prepared by Plasma Electrolytic Oxidation in Aluminate Electrolyte. Coatings. 2023; 13(5):848. https://doi.org/10.3390/coatings13050848
Chicago/Turabian StyleMaltanava, Hanna, Stevan Stojadinovic, Rastko Vasilic, Sergey Karpushenkov, Nikita Belko, Michael Samtsov, and Sergey Poznyak. 2023. "Photoluminescent Coatings on Zinc Alloy Prepared by Plasma Electrolytic Oxidation in Aluminate Electrolyte" Coatings 13, no. 5: 848. https://doi.org/10.3390/coatings13050848