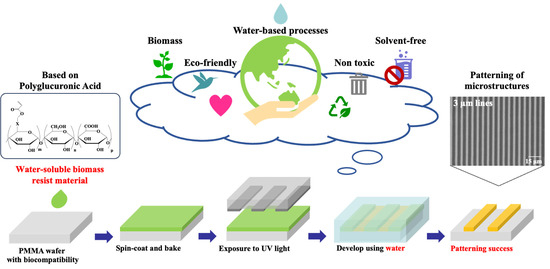
Water-Soluble Biomass Resist Materials Based on Polyglucuronic Acid for Eco-Friendly Photolithography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Water-Soluble Biomass Resist Materials
2.2. Exposure Sensitivity Measurement
2.3. Measurement of Film Thickness Uniformity
2.4. Water-Based Spin-Coating and Developing Processes in Eco-Friendly Photolithography
2.5. Mechanical Properties of the Films
2.6. Surface Zeta Potential Measurement
3. Results and Discussions
3.1. Exposure Sensitivity Measurement
3.2. Evaluation of Film Thickness Uniformity
3.3. Fine Processing Evaluation
3.4. Mechanical Properties of Films Results
3.5. Surface Zeta Potential Measurement Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadiku, M.N.O.; Abayomi, A.; Philip, O.A. Nanomanufacturing. In Emerging Technologies in Manufacturing; Springer: Cham, Switzerland, 2023; p. 201. [Google Scholar]
- Sharma, E.; Rathi, R.; Misharwal, J.; Sinhmar, B.; Kumari, S.; Dalal, J.; Kumar, A. Evolution in Lithography Techniques: Microlithography to Nanolithography. Nanomaterials 2022, 12, 2754. [Google Scholar] [CrossRef] [PubMed]
- Ermis, M.; Antmen, E.; Kuren, O.; Demirci, U.; Hasirci, V. A Cell Culture Chip with Transparent, Micropillar-Decorated Bottom for Live Cell Imaging and Screening of Breast Cancer Cells. Micromachines 2022, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Unno, N.; Tapio, M. Thermal nanoimprint lithography—A review of the process, mold fabrication, and material. Nanomaterials 2023, 13, 2031. [Google Scholar] [CrossRef]
- Nadine, S.; Chung, A.; Diltemiz, S.E.; Yasuda, B.; Lee, C.; Hosseini, V.; Karamikamkar, S.; Barros, N.R.; Mandal, K.; Advani, S.; et al. Advances in microfabrication technologies in tissue engineering and regenerative medicine. Artif. Organs 2022, 46, E211–E243. [Google Scholar] [CrossRef]
- Voldman, J.; Gray, M.L.; Schmidt, M.A. Microfabrication in biology and medicine. Annu. Rev. Biomed. Eng. 1999, 1, 401–425. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Hirano, A.; Nagasaki, Y.; Okano, T.; Horiike, Y.; Kataoka, K. Two-dimensional multiarray formation of hepatocyte spheroids on a microfabricated PEG-brush surface. ChemBioChem 2004, 5, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, P.; Bonabi, A.; Jokinen, V.; Sikanen, T. Simultaneous culturing of cell monolayers and spheroids on a single microfluidic device for bridging the gap between 2D and 3D cell assays in drug research. Adv. Funct. Mater. 2020, 30, 2000479. [Google Scholar] [CrossRef]
- Tamura, T.; Sakai, Y.; Nakazawa, K. Two-dimensional microarray of HepG2 spheroids using collagen/polyethylene glycol micropatterned chip. J. Mater. Sci. Mater. Med. 2008, 19, 2071–2077. [Google Scholar] [CrossRef]
- Thoma, C.R.; Zimmermann, M.; Agarkova, I.; Kelm, J.M.; Krek, W. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv. Drug Deliv. Rev. 2014, 69, 29–41. [Google Scholar] [CrossRef]
- Battista, S.; Guarnieri, D.; Borselli, C.; Zeppetelli, S.; Borzacchiello, A.; Mayol, L.; Gerbasio, D.; Keene, D.R.; Ambrosio, L.; Netti, P.A. The effect of matrix composition of 3D constructs on embryonic stem cell differentiation. Biomaterials 2005, 26, 6194–6207. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling physiological events in 2D vs. 3D cell culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Millet, M.; Messaoud, R.B.; Luthold, C.; Bordeleau, F. Coupling microfluidic platforms, microfabrication, and tissue engineered scaffolds to investigate tumor cells mechanobiology. Micromachines 2019, 10, 418. [Google Scholar] [CrossRef] [PubMed]
- Iandolo, D.; Pennacchio, F.A.; Mollo, V.; Rossi, D.; Dannhauser, D.; Cui, B.; Owens, R.M.; Santoro, F. Electron microscopy for 3D scaffolds–cell biointerface characterization. Adv. Biosyst. 2019, 3, 1800103. [Google Scholar] [CrossRef] [PubMed]
- Torisawa, Y.S.; Takagi, A.; Nashimoto, Y.; Yasukawa, T.; Shiku, H.; Matsue, T. A multicellular spheroid array to realize spheroid formation, culture, and viability assay on a chip. Biomaterials 2017, 28, 559–566. [Google Scholar] [CrossRef]
- Ramos-Rodriguez, D.H.; MacNeil, S.; Claeyssens, F.; Asencio, I.O. The Use of Microfabrication Techniques for the Design and Manufacture of Artificial Stem Cell Microenvironments for Tissue Regeneration. Bioengineering 2021, 8, 50. [Google Scholar] [CrossRef]
- Fukuda, J.; Khademhosseini, A.; Yeh, J.; Eng, G.; Cheng, J.; Farokhzad, O.C.; Langer, R. Micropatterned cell co-cultures using layer-by-layer deposition of extracellular matrix components. Biomaterials 2006, 27, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, X.; Tao, W.; Li, Y.; Du, Y.; Zhang, S. Micropatterned composite membrane guides oriented cell growth and vascularization for accelerating wound healing. Regen. Biomater. 2023, 10, rbac108. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, J.H.; Lee, H.J.; Lee, S.R. Tribology issues in nanoimprint lithography. J. Mech. Sci. Technol. 2010, 24, 5–12. [Google Scholar] [CrossRef]
- Ellenson, J.E.; Litt, L.C.; Rastegar, A. A study of template cleaning for nano-imprint lithography. Photomask Technol. 2007, 6730, 1821. [Google Scholar]
- Miura, S.; Yamagishi, R.; Miyazaki, R.; Yasuda, K.; Kawano, Y.; Yokoyama, Y.; Sugino, N.; Kameda, T.; Takei, S. Fabrication of high-resolution fine microneedles derived from hydrolyzed hyaluronic acid gels in vacuum environment imprinting using water permeable mold. Gels 2022, 8, 785. [Google Scholar] [CrossRef]
- Miura, S.; Yamagishi, R.; Sugino, N.; Yokoyama, Y.; Miyazaki, R.; Yasuda, K.; Ando, M.; Hachikubo, Y.; Murashita, T.; Kameda, T.; et al. Nanoimprint lithography and microinjection molding using gas-permeable hybrid mold for antibacterial nanostructures. J. Photopolym. Sci. Technol. 2023, 36, 183. [Google Scholar]
- Yamagishi, R.; Miura, S.; Yasuda, K.; Sugino, N.; Kameda, T.; Kawano, Y.; Yokoyama, Y.; Sugino, N.; Kameda, T.; Takei, S. Thermal nanoimprint lithography of sodium hyaluronate solutions with gas permeable inorganic hybrid mold for cosmetic and pharmaceutical applications. Appl. Phys. Express 2022, 15, 046502. [Google Scholar] [CrossRef]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.; Lima, R.; Minas, G. Properties and applications of PDMS for biomedical engineering: A review. J. Funct. Biomater. 2021, 13, 2. [Google Scholar] [CrossRef]
- Bat, E.; Lee, J.; Lau, U.Y.; Maynard, H.D. Trehalose glycopolymer resists allow direct writing of protein patterns by electron-beam lithography. Nat. Commun. 2015, 6, 6654. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.C.; Park, J.S.; Jha, R.K.; Kim, J.; Kim, J.; Kim, M.; Choi, J.; Kim, H.; Park, D.H.; Gogurla, N.; et al. Engineering Silk Protein to Modulate Polymorphic Transitions for Green Lithography Resists. ACS Appl. Mater. Interfaces 2022, 14, 56623–56634. [Google Scholar] [CrossRef]
- Aziz, T.; Ullah, A.; Ali, A.; Shabeer, M.; Shah, M.N.; Haq, F.; Iqbal, M.; Ullah, R.; Khan, F.U. Manufactures of bio-degradable and bio-based polymers for bio-materials in the pharmaceutical field. J. Appl. Polym. Sci. 2022, 139, e52624. [Google Scholar] [CrossRef]
- Gao, D.; Lv, J.; Lee, P.S. Natural polymer in soft electronics: Opportunities, challenges, and future prospects. Adv. Mater. 2022, 34, 2105020. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, S.G.; Marelli, B.; Lee, M.; Kim, T.; Oh, H.K.; Jeon, H.; Omenetto, F.G.; Kim, S. Eco-friendly photolithography using water-developable pure silk fibroin. RSC Adv. 2016, 6, 39330–39334. [Google Scholar] [CrossRef]
- Zhu, S.; Zeng, W.; Meng, Z.; Luo, W.; Ma, L.; Li, Y.; Lin, C.; Huang, Q.; Lin, Y.; Liu, X.Y. Using wool keratin as a basic resist material to fabricate precise protein patterns. Adv. Mater. 2019, 31, 1900870. [Google Scholar] [CrossRef] [PubMed]
- Hachikubo, Y.; Miura, S.; Yamagishi, R.; Ando, M.; Kobayashi, M.; Ota, T.; Amano, T.; Takei, S. Amylopectin-based eco-friendly photoresist material in water-developable lithography processes for surface micropatterns on polymer substrates. J. Photopolym. Sci. Technol. 2023, 36, 197. [Google Scholar]
- Richert, L.; Boulmedais, F.; Lavalle, P.; Mutterer, J.; Ferreux, E.; Decher, G.; Schaaf, P.; Voegel, J.C.; Picart, C. Improvement of stability and cell adhesion properties of polyelectrolyte multilayer films by chemical cross-linking. Biomacromolecules 2004, 5, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.; Jubeli, E.; Pungente, M.D.; Yagoubi, N. Biocompatibility of polymer-based biomaterials and medical devices–regulations, in vitro screening and risk-management. Biomater. Sci. 2018, 6, 2025–2053. [Google Scholar] [CrossRef] [PubMed]
- Takei, S.; Maki, H.; Sugahara, K.; Ito, K.; Hanabata, M. Inedible cellulose-based biomass resist material amenable to water-based processing for use in electron beam lithography. AIP Adv. 2015, 5, 077141. [Google Scholar] [CrossRef]
- Takei, S.; Oshima, A.; Oyama, T.G.; Ito, K.; Sugahara, K.; Kashiwakura, M.; Kozawa, T.; Tagawa, S.; Hanabata, M. Approach of natural polysaccharide to green resist polymers for EUV lithography. Jpn. J. Appl. Phys. 2014, 53, 116505. [Google Scholar] [CrossRef]
- Zheng, D.Y.; Chang, M.H.; Pan, C.L.; Oh-e, M. Effects of O2 plasma treatments on the photolithographic patterning of PEDOT: PSS. Coatings 2021, 11, 31. [Google Scholar] [CrossRef]
- Hamon, M.; Chen, Y.; Srivastava, P.; Chang, H.M.; Gupta, V.; Jin, L.; Yanagawa, N.; Hauser, P.V. Matrix Stiffness Influences Tubular Formation in Renal Tissue Engineering. Appl. Sci. 2023, 13, 4510. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255. [Google Scholar]
- Duan, X.; Li, Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miura, S.; Hachikubo, Y.; Yamagishi, R.; Ando, M.; Takei, S. Water-Soluble Biomass Resist Materials Based on Polyglucuronic Acid for Eco-Friendly Photolithography. Coatings 2023, 13, 2038. https://doi.org/10.3390/coatings13122038
Miura S, Hachikubo Y, Yamagishi R, Ando M, Takei S. Water-Soluble Biomass Resist Materials Based on Polyglucuronic Acid for Eco-Friendly Photolithography. Coatings. 2023; 13(12):2038. https://doi.org/10.3390/coatings13122038
Chicago/Turabian StyleMiura, Sayaka, Yuna Hachikubo, Rio Yamagishi, Mano Ando, and Satoshi Takei. 2023. "Water-Soluble Biomass Resist Materials Based on Polyglucuronic Acid for Eco-Friendly Photolithography" Coatings 13, no. 12: 2038. https://doi.org/10.3390/coatings13122038