Preparation of a Heterogeneous Catalyst CuO-Fe2O3/CTS-ATP and Degradation of Methylene Blue and Ciprofloxacin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
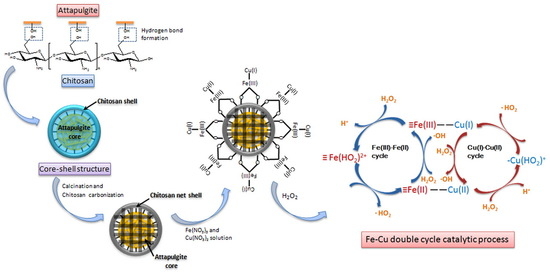
2.2. Preparation of CuO-Fe2O3/CTS-ATP
2.3. Characterization
2.4. Degradation Experiments of MB and CIP by the Catalysts
2.5. OH Measurement and Intermediate Product Determination
3. Results and Discussion
3.1. Characterization of Samples
3.2. MB and CIP Degradation by Prepared Catalysts
3.3. OH Concentrations Measurement
3.4. The Pathways of MB and CIP Degradation by CuO-Fe2O3/CTS-ATP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP | attapulgite |
| CTS | chitosan |
| ROS | refractory organics |
| MB | methylene blue |
| CIP | ciprofloxacin |
References
- Sun, Y.; Yu, I.K.; Tsang, D.C.; Fan, J.; Clark, J.H.; Luo, G.; Zhang, S.; Khan, E.; Graham, N.J. Tailored design of graphitic biochar for high-efficiency and chemical-free microwave-assisted removal of refractory organic contaminants. Chem. Eng. J. 2020, 398, 125505. [Google Scholar] [CrossRef]
- Ren, H.; Jin, X.; Li, C.; Li, T.; Liu, Y.; Zhou, R. Rosmarinic acid enhanced Fe(III)-mediated Fenton oxidation removal of organic pollutants at near neutral pH. Sci. Total Environ. 2020, 736, 139528. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, Z.; Zhu, L.; Wang, N.; Tang, H. Bisulfite-induced drastic enhancement of bisphenol A degradation in Fe3+-H2O2 Fenton system. Chem. Eng. J. 2018, 361, 1190–1197. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Zheng, J.; Yang, P.; Wu, X.; Cheng, C.; Li, J.; Tian, Y.; Wang, F. Improved organic pollutants removal and simultaneous electricity production via integrating Fenton process and dual rotating disk photocatalytic fuel cell system using bamboo charcoal cathode. Chem. Eng. J. 2019, 361, 1198–1206. [Google Scholar] [CrossRef]
- Kumar, N.; Mittal, H.; Parashar, V.; Ray, S.S.; Ngila, J.C. Efficient removal of rhodamine 6G dye from aqueous solution using nickel sulphide incorporated polyacrylamide grafted gum karaya bionanocomposite hydrogel. RSC Adv. 2016, 6, 21929–21939. [Google Scholar] [CrossRef]
- Zhang, G.-F.; Liu, X.; Zhang, S.; Pan, B.; Liu, M.-L. Ciprofloxacin derivatives and their antibacterial activities. Eur. J. Med. Chem. 2018, 146, 599–612. [Google Scholar] [CrossRef]
- Hassani, A.; Khataee, A.; Karaca, S.; Karaca, C.; Gholami, P. Sonocatalytic degradation of ciprofloxacin using synthesized TiO2 nanoparticles on montmorillonite. Ultrason. Sonochem. 2017, 35, 251–262. [Google Scholar] [CrossRef]
- Salma, A.; Thoröe-Boveleth, S.; Schmidt, T.C.; Tuerk, J. Dependence of transformation product formation on pH during photolytic and photocatalytic degradation of ciprofloxacin. J. Hazard. Mater. 2016, 313, 49–59. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kurade, M.B.; Kim, J.R.; Roh, H.-S.; Jeon, B.-H. Ciprofloxacin toxicity and its co-metabolic removal by a freshwater microalga Chlamydomonas mexicana. J. Hazard. Mater. 2017, 323, 212–219. [Google Scholar] [CrossRef]
- Luo, K.; Yang, Q.; Pang, Y.; Wang, D.; Li, X.; Lei, M.; Huang, Q. Unveiling the mechanism of biochar-activated hydrogen peroxide on the degradation of ciprofloxacin. Chem. Eng. J. 2019, 374, 520–530. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Huang, Y. Zeolitic imidazolate framework-8 derived nanoporous carbon as an effective and recyclable adsorbent for removal of ciprofloxacin antibiotics from water. J. Hazard. Mater. 2017, 321, 711–719. [Google Scholar] [CrossRef]
- Sun, Y.; Yue, Q.; Gao, B.; Gao, Y.; Xu, X.; Li, Q.; Wang, Y. Adsorption and cosorption of ciprofloxacin and Ni(II) on activated carbon-mechanism study. J. Taiwan Inst. Chem. Eng. 2014, 45, 681–688. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemoeller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, Y.; Geng, J.; Ren, H.; Xu, K.; Ding, L. Reduction of antibiotic resistance genes in municipal wastewater effluent by advanced oxidation processes. Sci. Total Environ. 2016, 550, 184–191. [Google Scholar] [CrossRef]
- Anjali, R.; Shanthakumar, S. Insights on the current status of occurrence and removal of antibiotics in wastewater by advanced oxidation processes. J. Environ. Manag. 2019, 246, 51–62. [Google Scholar] [CrossRef]
- Sharma, A.; Ahmad, J.; Flora, S. Application of advanced oxidation processes and toxicity assessment of transformation products. Environ. Res. 2018, 167, 223–233. [Google Scholar] [CrossRef]
- Liang, D.; Li, N.; An, J.; Ma, J.; Wu, Y.; Liu, H. Fenton-based technologies as efficient advanced oxidation processes for microcystin-LR degradation. Sci. Total Environ. 2020, 753, 141809. [Google Scholar] [CrossRef]
- Oluwole, A.O.; Omotola, E.O.; Olatunji, O.S. Pharmaceuticals and personal care products in water and wastewater: A review of treatment processes and use of photocatalyst immobilized on functionalized carbon in AOP degradation. BMC Chem. 2020, 14, 32. [Google Scholar] [CrossRef]
- Chaturvedi, N.K.; Katoch, S.S. Evaluation and comparison of Fenton-like oxidation with Fenton’s oxidation for hazardous methoxyanilines in aqueous solution. J. Ind. Eng. Chem. 2020, 92, 101–108. [Google Scholar] [CrossRef]
- Casado, C.; Moreno-SanSegundo, J.; De la Obra, I.; García, B.E.; Pérez, J.A.S.; Marugán, J. Mechanistic modelling of wastewater disinfection by the photo-Fenton process at circumneutral pH. Chem. Eng. J. 2020, 403, 126335. [Google Scholar] [CrossRef]
- Prakash, K.; Kumar, J.V.; Latha, P.; Kumar, P.S.; Karuthapandian, S. Fruitful fabrication of CDs on GO/g-C3N4 sheets layers: A carbon amalgamation for the remediation of carcinogenic pollutants. J. Photochem. Photobiol. A Chem. 2018, 370, 94–104. [Google Scholar] [CrossRef]
- Wu, Q.; Siddique, M.S.; Yu, W. Iron-nickel bimetallic metal-organic frameworks as bifunctional Fenton-like catalysts for enhanced adsorption and degradation of organic contaminants under visible light: Kinetics and mechanistic studies. J. Hazard. Mater. 2020, 401, 123261. [Google Scholar] [CrossRef]
- Xue, C.; Peng, Y.; Chen, A.; Peng, L.; Luo, S. Drastically inhibited nZVI-Fenton oxidation of organic pollutants by cysteine: Multiple roles in the nZVI/O2/hv system. J. Colloid Interf. Sci. 2020, 582, 22–29. [Google Scholar] [CrossRef]
- Gao, C.; Chen, S.; Quan, X.; Yu, H.; Zhang, Y. Enhanced Fenton-like catalysis by iron-based metal organic frameworks for degradation of organic pollutants. J. Catal. 2017, 356, 125–132. [Google Scholar] [CrossRef]
- Guo, H.; Li, Z.; Zhang, Y.; Jiang, N.; Wang, H.; Li, J. Degradation of chloramphenicol by pulsed discharge plasma with heterogeneous Fenton process using Fe3O4 nanocomposites. Sep. Purif. Technol. 2020, 253, 117540. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, D.; Zhang, Q.; Cui, R.; Hassan, M.; Dong, L.; Li, J.; He, Y. Single Mn atom anchored on N-doped porous carbon as highly efficient Fenton-like catalyst for the degradation of organic contaminants. Appl. Catal. B Environ. 2020, 279, 119363. [Google Scholar] [CrossRef]
- Jia, C.; Mi, Y.; Liu, Z.; Zhou, W.; Gao, H.; Zhang, S.; Lu, R. Attapulgite modified with covalent organic frameworks as the sorbent in dispersive solid phase extraction for the determination of pyrethroids in environmental water samples. Microchem. J. 2019, 153, 104522. [Google Scholar] [CrossRef]
- Yao, D.; Shi, Y.; Pan, H.; Zhong, D.; Hou, H.; Wu, X.; Chen, J.; Wang, L.; Hu, Y.; Crittenden, J.C. Promotion mechanism of natural clay colloids in the adsorption of arsenite on iron oxide particles in water. Chem. Eng. J. 2019, 392, 123637. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, Z.; Hao, R.; Xu, H.; Huang, C. Rapid adsorption and reductive degradation of Naphthol Green B from aqueous solution by Polypyrrole/Attapulgite composites supported nanoscale zero-valent iron. J. Hazard. Mater. 2019, 371, 8–17. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, H.; Peng, F.; Yang, H.; Xiong, L.; Wang, C.; Huang, C.; Chen, X.; Ma, L. Effects of Cu/Fe ratio on structure and performance of attapulgite supported CuFeCo-based catalyst for mixed alcohols synthesis from syngas. Appl. Catal. A Gen. 2015, 503, 51–61. [Google Scholar] [CrossRef]
- Han, D.; Zhao, H.; Gao, L.; Qin, Z.; Ma, J.; Han, Y.; Jiao, T. Preparation of carboxymethyl chitosan/phytic acid composite hydrogels for rapid dye adsorption in wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127355. [Google Scholar] [CrossRef]
- Iber, B.T.; Okomoda, V.T.; Rozaimah, S.A.; Kasan, N.A. Eco-friendly approaches to aquaculture wastewater treatment: Assessment of natural coagulants vis-a-vis chitosan. Bioresour. Technol. Rep. 2021, 15, 100702. [Google Scholar] [CrossRef]
- Vedula, S.S.; Yadav, G.D. Wastewater treatment containing methylene blue dye as pollutant using adsorption by chitosan lignin membrane: Development of membrane, characterization and kinetics of adsorption. J. Indian Chem. Soc. 2021, 99, 100263. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, Y.; Ma, L.; Yu, S.; Li, J. Preparation and characterization of chitosan/purified attapulgite composite for sharp adsorption of humic acid from aqueous solution at low temperature. J. Taiwan Inst. Chem. Eng. 2017, 78, 96–103. [Google Scholar] [CrossRef]
- Liang, X.X.; Ouyanga, X.K.; Wanga, S.; Yanga, L.Y.; Huanga, F.; Jia, C.; Chenb, X. Efficient adsorption of Pb(II) from aqueous solutions using aminopropyltriethoxysilane-modified magnetic attapulgite@chitosan (APTS-Fe3O4/APT@CS) composite hydrogel beads. Int. J. Biol. Macromol. 2019, 137, 741–750. [Google Scholar] [CrossRef]
- Pan, D.; Fan, Q.; Fan, F.; Tang, Y.; Zhang, Y.; Wu, W. Removal of uranium contaminant from aqueous solution by chitosan@attapulgite composite. Sep. Purif. Technol. 2017, 177, 86–93. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, M.; Chen, D. Production of three-dimensional porous polydopamine-functionalized attapulgite/chitosan aerogel for uranium(VI) adsorption. J. Radioanal. Nucl. Chem. Artic. 2018, 316, 635–647. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Q.; Feng, L.; Xiong, Q.; Chen, J. Preparation and adsorption characters of Cu(II)-imprinted chitosan/attapulgite polymer. Korean J. Chem. Eng. 2014, 31, 821–827. [Google Scholar] [CrossRef]
- Krishnan, A.; Vishwanathan, P.V.; Mohan, A.C.; Panchami, R.; Viswanath, S.; Krishnan, A.V. Tuning of photocatalytic performance of CeO2-Fe2O3 composite by Sn-doping for the effective degradation of methlene blue (MB) and methyl orange (MO) dyes. Surf. Interfaces 2020, 22, 100808. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Xie, X.; Zhu, J.; Li, R.; Qin, T. Removal of Norfloxacin from aqueous solution by clay-biochar composite prepared from potato stem and natural attapulgite. Colloids Surf. A Physicochem. Eng. Asp. 2017, 514, 126–136. [Google Scholar] [CrossRef]
- Zhang, T.; Dong, L.; Du, J.; Qian, C.; Wang, Y. CuO and CeO2 assisted Fe2O3/attapulgite catalyst for heterogeneous Fenton-like oxidation of methylene blue. RSC Adv. 2020, 10, 23431–23439. [Google Scholar] [CrossRef]
- Lei, Y.; Luo, Y.; Li, X.; Lu, J.; Mei, Z.; Peng, W.; Chen, R.; Chen, K.; Chen, D.; He, D. The role of samarium on Cu/Al2O3 catalyst in the methanol steam reforming for hydrogen production. Catal. Today 2018, 307, 162–168. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, R.; Wang, C.; Zhou, G.; Hua, C.; Cao, Y.; Song, Z. Novel environmental-friendly nano-composite magnetic attapulgite functionalized by chitosan and EDTA for cadmium (II) removal. J. Alloys Compd. 2019, 817, 153286. [Google Scholar] [CrossRef]
- Ismail, I.; Abdallah, B.; Abou-Kharroub, M.; Mrad, O. XPS and RBS investigation of TiNxOy films prepared by vacuum arc discharge. Nucl. Instrum. Methods Phys. Res. B Beam Interact. Mater. At. 2012, 271, 102–106. [Google Scholar] [CrossRef]
- Guo, H.-J.; Li, Q.-L.; Zhang, H.-R.; Xiong, L.; Peng, F.; Yao, S.-M.; Chen, X.-D. Attapulgite supported Cu-Fe-Co based catalyst combination system for CO hydrogenation to lower alcohols. J. Fuel Chem. Technol. 2019, 47, 1346–1356. [Google Scholar] [CrossRef]
- Qu, J.; Che, T.; Shi, L.; Lu, Q.; Qi, S. A novel magnetic silica supported spinel ferrites NiFe2O4 catalyst for heterogeneous Fenton-like oxidation of rhodamine B. Chin. Chem. Lett. 2019, 30, 1198–1203. [Google Scholar] [CrossRef]
- Qin, X.; Wang, Z.; Guo, C.; Guo, R.; Lv, Y.; Li, M. Fulvic acid degradation in Fenton-like system with bimetallic magnetic carbon aerogel Cu-Fe@CS as catalyst: Response surface optimization, kinetic and mechanism. J. Environ. Manag. 2022, 306, 114500. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, D.; Yao, Z.; Jiang, Z. Revealing the enhancing mechanisms of Fe–Cu bimetallic catalysts for the Fenton-like degradation of phenol. Chemosphere 2022, 289, 133195. [Google Scholar] [CrossRef]
- Qi, H.; Pan, G.; Shi, X.; Sun, Z. Cu–Fe–FeC3@nitrogen-doped biochar microsphere catalyst derived from CuFe2O4@chitosan for the efficient removal of amoxicillin through the heterogeneous electro-Fenton process. Chem. Eng. J. 2022, 434, 134675. [Google Scholar] [CrossRef]
- Moztahida, M.; Lee, D.S. Photocatalytic degradation of methylene blue with P25/graphene/polyacrylamide hydrogels: Optimization using response surface methodology. J. Hazard. Mater. 2020, 400, 123314. [Google Scholar] [CrossRef]
- Zhang, T.; Qian, C.; Guo, P.; Gan, S.; Dong, L.; Bai, G.; Guo, Q. Guo A Novel Reduced Graphene Oxide-Attapulgite (RGO-ATP) Supported Fe2O3 Catalyst for Heterogeneous Fenton-like Oxidation of Ciprofloxacin: Degradation Mechanism and Pathway. Catalysts 2020, 10, 189. [Google Scholar] [CrossRef] [Green Version]









| Samples | BET Surface Area/m2·g−1 | Pore Volume/cm3·g−1 | Pore Size/nm |
|---|---|---|---|
| ATP | 93.620 | 21.509 | 0.227 |
| Fe2O3/CTS-ATP | 21.997 | 0.061 | 11.013 |
| CuO-Fe2O3/CTS-ATP | 23.23 | 0.095 | 14.271 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Li, W.; Guo, Q.; Wang, Y.; Li, C. Preparation of a Heterogeneous Catalyst CuO-Fe2O3/CTS-ATP and Degradation of Methylene Blue and Ciprofloxacin. Coatings 2022, 12, 559. https://doi.org/10.3390/coatings12050559
Zhang T, Li W, Guo Q, Wang Y, Li C. Preparation of a Heterogeneous Catalyst CuO-Fe2O3/CTS-ATP and Degradation of Methylene Blue and Ciprofloxacin. Coatings. 2022; 12(5):559. https://doi.org/10.3390/coatings12050559
Chicago/Turabian StyleZhang, Ting, Wenhui Li, Qiyang Guo, Yi Wang, and Chunlei Li. 2022. "Preparation of a Heterogeneous Catalyst CuO-Fe2O3/CTS-ATP and Degradation of Methylene Blue and Ciprofloxacin" Coatings 12, no. 5: 559. https://doi.org/10.3390/coatings12050559