Simultaneous Precipitation and Electrodeposition of Hydroxyapatite Coatings at Different Temperatures on Various Metal Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
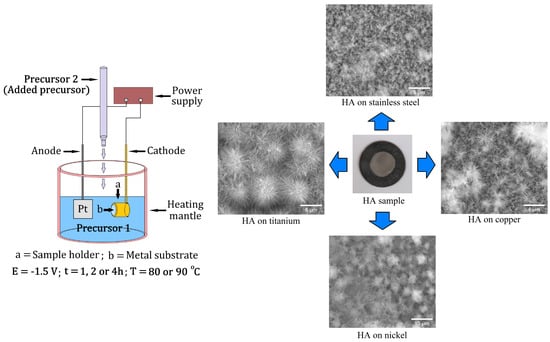
2.2. Electrochemical Deposition
2.3. Physico-Chemical Characterization
3. Results and Discussion
3.1. The First Comparative Study
3.2. The Second Comparative Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, T.T.; Ling, L.; Lin, M.C.; Peng, H.K.; Ren, H.T.; Lou, C.W.; Lin, J.H. Recent advances in multifunctional hydroxyapatite coating by electrochemical deposition. J. Mater. Sci. 2020, 55, 6352–6374. [Google Scholar] [CrossRef]
- Alizadeh-Osgouei, M.; Li, Y.; Wen, C. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioact. Mater. 2019, 4, 22–36. [Google Scholar] [CrossRef]
- Li, Z.; Chu, D.; Gao, Y.; Jin, L.; Zhang, X.; Cui, W.; Li, J. Biomimicry, biomineralization, and bioregeneration of bone using advanced three-dimensional fibrous hydroxyapatite scaffold. Mater. Today Adv. 2019, 3, 100014. [Google Scholar] [CrossRef]
- Sattar, T.; Manzoor, T.; Khalid, F.A.; Akmal, M.; Saeed, G. Improved in vitro bioactivity and electrochemical behavior of hydroxyapatite-coated NiTi shape memory alloy. J. Mater. Sci. 2019, 54, 7300–7306. [Google Scholar] [CrossRef]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Wagoner Johnson, A.J. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef]
- Lin, L.; Chow, K.L.; Leng, Y. Study of hydroxyapatite osteoinductivity with an osteogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res.—Part A 2009, 89, 326–335. [Google Scholar] [CrossRef]
- Aktuğ, S.L.; Durdu, S.; Yalçın, E.; Çavuşoğlu, K.; Usta, M. Bioactivity and biocompatibility of hydroxyapatite-based bioceramic coatings on zirconium by plasma electrolytic oxidation. Mater. Sci. Eng. C 2017, 71, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Albahadli, N.A.; Ajeel, S. Hydroxyapatite coating improved corrosion resistance of 316L stainless steel used for surgical implants applications. Eng. Technol. J. 2008, 26, 958–964. [Google Scholar]
- Qadir, M.; Li, Y.; Wen, C. Ion-substituted calcium phosphate coatings by physical vapor deposition magnetron sputtering for biomedical applications: A review. Acta Biomater. 2019, 89, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Kim, S.Y.; Yi, Y.J.; Lee, B.K.; Kim, Y.K. Hydroxyapatite-coated implant: Clinical prognosis assessment via a retrospective followup study for the average of 3 years. J. Adv. Prosthodont. 2018, 10, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Ghiasi, B.; Sefidbakht, Y.; Rezaei, M. Hydroxyapatite for biomedicine and drug delivery. In Nanomaterials for Advanced Biological Applications; Rahmandoust, M., Ayatollahi, M.R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 85–120. ISBN 9783030108342. [Google Scholar]
- Bakan, F. Gene delivery by hydroxyapatite and calcium phosphate nanoparticles: A review of novel and recent applications. In Hydroxyapatite—Advances in Composite Nanomaterials, Biomedical Applications and Its Technological Facets; Thirumalai, J., Ed.; InTech Croatia: Rijeka, Croatia, 2018; pp. 157–176. [Google Scholar]
- Abu Bakar, M.S.; Cheng, M.H.W.; Tang, S.M.; Yu, S.C.; Liao, K.; Tan, C.T.; Khor, K.A.; Cheang, P. Tensile properties, tension-tension fatigue and biological response of polyetheretherketone-hydroxyapatite composites for load-bearing orthopedic implants. Biomaterials 2003, 24, 2245–2250. [Google Scholar] [CrossRef]
- Mihailescu, N.; Stan, G.E.; Duta, L.; Chifiriuc, M.C.; Bleotu, C.; Sopronyi, M.; Luculescu, C.; Oktar, F.N.; Mihailescu, I.N. Structural, compositional, mechanical characterization and biological assessment of bovine-derived hydroxyapatite coatings reinforced with MgF2 or MgO for implants functionalization. Mater. Sci. Eng. C 2016, 59, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Stefan, L.G.; Abrudeanu, M.; Iosub, I.; Plaiasu, A.G.; Dinu, A.; Mihalache, M. Electrodeposition of hydroxyapatite coatings on stainless steel 316 L. Sci. Bull. Automot. Ser. 2009, A, 129–134. [Google Scholar]
- Harun, W.S.W.; Asri, R.I.M.; Alias, J.; Zulkifli, F.H.; Kadirgama, K.; Ghani, S.A.C.; Shariffuddin, J.H.M. A comprehensive review of hydroxyapatite-based coatings adhesion on metallic biomaterials. Ceram. Int. 2018, 44, 1250–1268. [Google Scholar] [CrossRef]
- Arabnejad, S.; Burnett Johnston, R.; Pura, J.A.; Singh, B.; Tanzer, M.; Pasini, D. High-strength porous biomaterials for bone replacement: A strategy to assess the interplay between cell morphology, mechanical properties, bone ingrowth and manufacturing constraints. Acta Biomater. 2016, 30, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metals for implants: A review. J. Alloys Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef] [Green Version]
- Yılmaz, E.; Gökçe, A.; Findik, F.; Gulsoy, H.O.; İyibilgin, O. Mechanical properties and electrochemical behavior of porous Ti-Nb biomaterials. J. Mech. Behav. Biomed. Mater. 2018, 87, 59–67. [Google Scholar] [CrossRef]
- De Sena, L.Á.; De Andrade, M.C.; Rossi, A.M.; De Almeida Soares, G. Hydroxyapatite deposition by electrophoresis on titanium sheets with different surface finishing. J. Biomed. Mater. Res. 2002, 60, 1–7. [Google Scholar] [CrossRef]
- Spoerke, E.D.; Murray, N.G.; Li, H.; Brinson, L.C.; Dunand, D.C.; Stupp, S.I. A bioactive titanium foam scaffold for bone repair. Acta Biomater. 2005, 1, 523–533. [Google Scholar] [CrossRef]
- Isa, N.N.C.; Mohd, Y.; Yury, N. Electrodeposition of hydroxyapatite (HAp) coatings on etched titanium mesh substrate. In CHUSER 2012—2012 IEEE Colloquium on Humanities, Science and Engineering (CHUSER); IEEE Xplore: Piscataway, NJ, USA, 2012; pp. 771–775. [Google Scholar] [CrossRef]
- Vo, T.H.; Le, T.D.; Pham, T.N.; Nguyen, T.T.; Nguyen, T.P.; Thanh Dinh, T.M. Electrodeposition and characterization of hydroxyapatite coatings doped by Sr2+, Mg2+, Na+ and F- on 316L stainless steel. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 045001. [Google Scholar] [CrossRef]
- Bryington, M.S.; Hayashi, M.; Kozai, Y.; Vandeweghe, S.; Andersson, M.; Wennerberg, A.; Jimbo, R. The influence of nano hydroxyapatite coating on osseointegration after extended healing periods. Dent. Mater. 2013, 29, 514–520. [Google Scholar] [CrossRef]
- Gambardella, A.; Bianchi, M.; Kaciulis, S.; Mezzi, A.; Brucale, M.; Cavallini, M.; Herrmannsdoerfer, T.; Chanda, G.; Uhlarz, M.; Cellini, A.; et al. Magnetic hydroxyapatite coatings as a new tool in medicine: A scanning probe investigation. Mater. Sci. Eng. C 2016, 62, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Hiromoto, S.; Inoue, M.; Taguchi, T.; Yamane, M.; Ohtsu, N. In vitro and in vivo biocompatibility and corrosion behaviour of a bioabsorbable magnesium alloy coated with octacalcium phosphate and hydroxyapatite. Acta Biomater. 2015, 11, 520–530. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate coatings on magnesium and its biodegradable alloys. Acta Biomater. 2014, 10, 2919–2934. [Google Scholar] [CrossRef] [PubMed]
- Suvorova, E.I.; Klechkovskaya, V.V.; Bobrovsky, V.V.; Khamchukov, Y.D.; Klubovich, V.V. Nanostructure of plasma-sprayed hydroxyapatite coating. Crystallogr. Rep. 2003, 48, 872–877. [Google Scholar] [CrossRef]
- Habibovic, P.; Li, J.; Van Der Valk, C.M.; Meijer, G.; Layrolle, P.; Van Blitterswijk, C.A.; De Groot, K. Biological performance of uncoated and octacalcium phosphate-coated Ti6Al4V. Biomaterials 2005, 26, 23–36. [Google Scholar] [CrossRef]
- Xue, J.; Farris, A.; Wang, Y.; Yeh, W.; Romany, C.; Guest, J.K.; Grayson, W.L.; Hall, A.S.; Weihs, T.P. Electrodeposition of hydroxyapatite on a metallic 3D-woven bioscaffold. Coatings 2020, 10, 715. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Ahangari, A.S.; Zadeh, H.; Vaezi, M.R.; Mozafari, M. A new double-layer hydroxyapatite/alumina-silica coated titanium implants using plasma spray technique. Surf. Coat. Technol. 2018, 352, 474–482. [Google Scholar] [CrossRef]
- Huang, H.; Lan, P.H.; Zhang, Y.Q.; Li, X.K.; Zhang, X.; Yuan, C.F.; Zheng, X.-B.; Guo, Z. Surface characterization and in vivo performance of plasma-sprayed hydroxyapatite-coated porous Ti6Al4V implants generated by electron beam melting. Surf. Coat. Technol. 2015, 283, 80–88. [Google Scholar] [CrossRef]
- Peng, P.; Kumar, S.; Voelcker, N.H.; Szili, E.; Smart, R.S.C.; Griesser, H.J. Thin calcium phosphate coatings on titanium by electrochemical deposition in modified simulated body fluid. J. Biomed. Mater. Res.—Part A 2006, 76, 347–355. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Hassan, M.A.; Ghani, S.A.C.; Buyong, Z. A review of hydroxyapatite-based coating techniques: Sol-gel and electrochemical depositions on biocompatible metals. J. Mech. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catauro, M.; Papale, F.; Bollino, F. Characterization and biological properties of TiO2/PCL hybrid layers prepared via sol-gel dip coating for surface modification of titanium implants. J. Non. Cryst. Solids 2015, 415, 9–15. [Google Scholar] [CrossRef]
- Yigit, O.; Dikici, B.; Ozdemir, N. Hydrothermal synthesis of nanocrystalline hydroxyapatite–graphene nanosheet on Ti-6Al-7Nb: Mechanical and in vitro corrosion performance. J. Mater. Sci. Mater. Med. 2021, 32, 40. [Google Scholar] [CrossRef] [PubMed]
- Kien, P.T.; Quan, T.N.; Tuyet Anh, L.H. Coating characteristic of hydroxyapatite on titanium substrates via hydrothermal treatment. Coatings 2021, 11, 1226. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Surmeneva, M.A.; Surmenev, R.A.; Depla, D. Structural evolution and growth mechanisms of RF-magnetron sputter-deposited hydroxyapatite thin films on the basis of unified principles. Appl. Surf. Sci. 2017, 425, 497–506. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Komarova, E.G.; Sharkeev, Y.P.; Ugodchikova, A.V.; Mushtovatova, L.S.; Karpova, M.R.; Sheikin, V.V.; Litvinova, L.S.; Khlusov, I.A. Zn-, Cu- or Ag-incorporated micro-arc coatings on titanium alloys: Properties and behavior in synthetic biological media. Surf. Coat. Technol. 2019, 369, 52–68. [Google Scholar] [CrossRef]
- Khandelwal, H.; Singh, G.; Agrawal, K.; Prakash, S.; Agarwal, R.D. Characterization of hydroxyapatite coating by pulse laser deposition technique on stainless steel 316 L by varying laser energy. Appl. Surf. Sci. 2013, 265, 30–35. [Google Scholar] [CrossRef]
- Ehsani, N.; Sorrell, C.C.; Ruys, A.J. Hydroxyapatite matrix composites by hot isostatic pressing: Part 2. Zirconia fibre and powder reinforced. J. Biomim. Biomater. Tissue Eng. 2012, 15, 85–100. [Google Scholar] [CrossRef]
- Yılmaz, E.; Gökçe, A.; Findik, F.; Gulsoy, H.O. Metallurgical properties and biomimetic HA deposition performance of Ti-Nb PIM alloys. J. Alloys Compd. 2018, 746, 301–313. [Google Scholar] [CrossRef]
- Bolelli, G.; Bellucci, D.; Cannillo, V.; Gadow, R.; Killinger, A.; Lusvarghi, L.; Müller, P.; Sola, A. Comparison between suspension plasma sprayed and high velocity suspension flame sprayed bioactive coatings. Surf. Coat. Technol. 2015, 280, 232–249. [Google Scholar] [CrossRef]
- Klyui, N.I.; Temchenko, V.P.; Gryshkov, A.P.; Dubok, V.A.; Shynkaruk, A.V.; Lyashenko, B.A.; Barynov, S.M. Properties of the hydroxyapatite coatings obtained by gas-detonation deposition onto titanium substrates. Funct. Mater. 2011, 18, 285–292. [Google Scholar]
- Tahmasbi Rad, A.; Solati-Hashjin, M.; Osman, N.A.A.; Faghihi, S. Improved bio-physical performance of hydroxyapatite coatings obtained by electrophoretic deposition at dynamic voltage. Ceram. Int. 2014, 40, 12681–12691. [Google Scholar] [CrossRef] [Green Version]
- Dinh, T.M.T.; Nguyen, T.T.; Pham, T.N.; Nguyen, T.P.; Nguyen, T.T.T.; Hoang, T.; Grossin, D.; Bertrand, G.; Drouet, C. Electrodeposition of HAp coatings on Ti6Al4V alloy and its electrochemical behavior in simulated body fluid solution. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 025008. [Google Scholar] [CrossRef]
- Yılmaz, E.; Çakıroğlu, B.; Gökçe, A.; Findik, F.; Gulsoy, H.O.; Gulsoy, N.; Mutlu, Ö.; Özacar, M. Novel hydroxyapatite/graphene oxide/collagen bioactive composite coating on Ti16Nb alloys by electrodeposition. Mater. Sci. Eng. C 2019, 101, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Bakin, B.; Koc Delice, T.; Tiric, U.; Birlik, I.; Ak Azem, F. Bioactivity and corrosion properties of magnesium-substituted CaP coatings produced via electrochemical deposition. Surf. Coat. Technol. 2016, 301, 29–35. [Google Scholar] [CrossRef]
- San, H.; Hu, J.; Zhang, Y.; Han, J.; Tang, S. Formation and in vitro mineralization of electrochemically deposited coatings prepared on micro-arc oxidized titanium alloy. J. Appl. Electrochem. 2019, 49, 485–501. [Google Scholar] [CrossRef]
- Wu, P.P.; Zhang, Z.Z.; Xu, F.J.; Deng, K.K.; Nie, K.B.; Gao, R. Effect of duty cycle on preparation and corrosion behavior of electrodeposited calcium phosphate coatings on AZ91. Appl. Surf. Sci. 2017, 426, 418–426. [Google Scholar] [CrossRef]
- Katić, J.; Metikoš-Huković, M.; Škapin, S.D.; Petravić, M.; Varašanec, M. The potential-assisted deposition as valuable tool for producing functional apatite coatings on metallic materials. Electrochim. Acta 2014, 127, 173–179. [Google Scholar] [CrossRef]
- Bucur, A.I.; Linul, E.; Taranu, B.O. Hydroxyapatite coatings on Ti substrates by simultaneous precipitation and electrodeposition. Appl. Surf. Sci. 2020, 527, 146820. [Google Scholar] [CrossRef]
- Eliaz, N.; Eliyahu, M. Electrochemical processes of nucleation and growth of hydroxyapatite on titanium supported by real-time electrochemical atomic force microscopy. J. Biomed. Mater. Res. Part A 2006, 80, 621–634. [Google Scholar] [CrossRef]
- Isa, N.N.C.; Mohd, Y.; Yury, N. Electrochemical deposition and characterization of hydroxyapatite (HAp) on titanium substrate. APCBEE Procedia 2012, 3, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Taranu, B.O.; Bucur, A.I.; Sebarchievici, I. Three-step procedure for the deposition of hydroxyapatite coatings. J. Coat. Technol. Res. 2020, 17, 1075–1082. [Google Scholar] [CrossRef]
- Bucur, A.I.; Bucur, R.A.; Szabadai, Z.; Mosoarca, C.; Linul, P.A. Influence of small concentration addition of tartaric acid on the 220 °C hydrothermal synthesis of hydroxyapatite. Mater. Charact. 2017, 132, 76–82. [Google Scholar] [CrossRef]
- Gopi, D.; Bhuvaneshwari, N.; Indira, J.; Kanimozhi, K.; Kavitha, L. A novel green template assisted synthesis of hydroxyapatite nanorods and their spectral characterization. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2013, 107, 196–202. [Google Scholar] [CrossRef]
- Valério, A.; Morelhão, S.L. Usage of Scherrer’s formula in X-ray diffraction analysis of size distribution in systems of monocrystalline nanoparticles. arXiv 2019, arXiv:1911.00701. [Google Scholar]
- Rabiei, M.; Palevicius, A.; Monshi, A.; Nasiri, S.; Vilkauskas, A.; Janusas, G. Comparing methods for calculating nano crystal size of natural hydroxyapatite using X-ray diffraction. Nanomaterials 2020, 10, 1627. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, M.; Stefaniuk, T.; Pniewski, J. The technique of measurement of intraocular lens surface roughness using atomic force microscopy. Interdiscip. J. Eng. Sci. 2014, 2, 21–25. [Google Scholar]
- Rajesh Kumar, B.; Subba Rao, T. AFM studies on surface morphology, topography and texture of nanostructured zinc aluminum oxide thin films. Dig. J. Nanomater. Biostruct. 2012, 7, 1881–1889. [Google Scholar]
- Roşu, R.A.; Şerban, V.-A.; Bucur, A.I.; Dragoş, U. Deposition of titanium nitride and hydroxyapatite-based biocompatible composite by reactive plasma spraying. Appl. Surf. Sci. 2012, 258, 3871–3876. [Google Scholar] [CrossRef]
- García, C.; Ceré, S.; Durán, A. Bioactive coatings deposited on titanium alloys. J. Non. Cryst. Solids 2006, 352, 3488–3495. [Google Scholar] [CrossRef]
- Niinomi, M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A 2002, 33A, 477–486. [Google Scholar] [CrossRef]
- Qiu, D.; Wang, A.; Yin, Y. Characterization and corrosion behavior of hydroxyapatite/zirconia composite coating on NiTi fabricated by electrochemical deposition. Appl. Surf. Sci. 2010, 257, 1774–1778. [Google Scholar] [CrossRef]
- Adhitya, K. Electrophoretic deposition of calcium phosphate ceramics over duplex stainless steel (S2205) and its characterization. Int. Res. J. Eng. Technol. 2020, 7, 1905–1909. [Google Scholar]
- Durairaj, R.B.; Ramachandran, S. Corrosion characteristics of hydroxyapatite coated titanium substrate for biomedical applications. Int. J. Chem. Sci. 2016, 14, 2157–2163. [Google Scholar]
- Huang, S.; Zhou, K.; Huang, B.; Li, Z.; Zhu, S.; Wang, G. Preparation of an electrodeposited hydroxyapatite coating on titanium substrate suitable for in-vivo applications. J. Mater. Sci. Mater. Med. 2008, 19, 437–442. [Google Scholar] [CrossRef]
- Gopi, D.; Collins Arun Prakash, V.; Kavitha, L.; Kannan, S.; Bhalaji, P.R.; Shinyjoy, E.; Ferreira, J.M.F. A facile electrodeposition of hydroxyapatite onto borate passivated surgical grade stainless steel. Corros. Sci. 2011, 53, 2328–2334. [Google Scholar] [CrossRef]
- Thom, N.T.; Nam, P.T.; Phuong, N.T.; Thi, D.; Thanh, M. Investigation of the condition to synthesize HAp/CNTs coatings on 316LSS. Vietnam J. Sci. Technol. 2018, 56, 50–62. [Google Scholar]
- Ghosh, R.; Swart, O.; Westgate, S.; Miller, B.L.; Yates, M.Z. Antibacterial copper-hydroxyapatite composite coatings via electrochemical synthesis. Langmuir 2019, 35, 5957–5966. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, X.; Mao, H.; Li, T.; Zhao, R.; Yan, Y.; Pang, X. Osteoblastic cell responses and antibacterial efficacy of Cu/Zn co-substituted hydroxyapatite coatings on pure titanium using electrodeposition method. RSC Adv. 2015, 5, 17076–17086. [Google Scholar] [CrossRef]
- Huang, Y.; Hao, M.; Nian, X.; Qiao, H.; Zhang, X.; Zhang, X.; Song, G.; Guo, J.; Pang, X.; Zhang, H. Strontium and copper co-substituted hydroxyapatite-based coatings with improved antibacterial activity and cytocompatibility fabricated by electrodeposition. Ceram. Int. 2016, 42, 11876–11888. [Google Scholar] [CrossRef]
- Lobo, A.; Marciano, F.R.; Matsushima, J.T.; Ramos, S.C.; Corat, E.J. Influence of temperature and time for direct hydroxyapatite electrodeposition on superhydrophilic vertically aligned carbon nanotube films. J. Nanomed. Nanotechnol. 2011, 6, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Savino, K.; Yates, M.Z. Coating of hydroxyapatite films on metal substrates by seeded hydrothermal deposition. Surf. Coat. Technol. 2011, 205, 3975–3986. [Google Scholar] [CrossRef]
- Thanh, D.T.M.; Nam, P.T.; Phuong, N.T.; Que, L.X.; Van Anh, N.; Hoang, T.; Lam, T.D. Controlling the electrodeposition, morphology and structure of hydroxyapatite coating on 316L stainless steel. Mater. Sci. Eng. C 2013, 33, 2037–2045. [Google Scholar] [CrossRef]
- Gopi, D.; Indira, J.; Kavitha, L. A comparative study on the direct and pulsed current electrodeposition of hydroxyapatite coatings on surgical grade stainless steel. Surf. Coat. Technol. 2012, 206, 2859–2869. [Google Scholar] [CrossRef]
- Büyüksağiş, A.; Bulut, E.; Kayalı, Y. Corrosion behaviors of hydroxyapatite coated by electrodeposition method of Ti6Al4V, Ti and AISI 316L SS substrates. Prot. Met. Phys. Chem. Surf. 2013, 49, 776–787. [Google Scholar] [CrossRef]
- Chakraborty, R.; Sengupta, S.; Saha, P.; Das, K.; Das, S. Synthesis of calcium hydrogen phosphate and hydroxyapatite coating on SS316 substrate through pulsed electrodeposition. Mater. Sci. Eng. C 2016, 69, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Saha, P. A comparative study on surface morphology and electrochemical behaviour of hydroxyapatite-calcium hydrogen phosphate composite coating synthesized in-situ through electro chemical process under various deposition conditions. Surf. Interfaces 2018, 12, 160–167. [Google Scholar] [CrossRef]
- Chennah, A.; Naciri, Y.; Ahsaine, H.A.; Taoufyq, A.; Bakiz, B.; Bazzi, L.; Guinneton, F.; Gavarri, J.R.; Benlhachemi, A. Electrocatalytic properties of hydroxyapatite thin films electrodeposited on stainless steel substrates. Mediterr. J. Chem. 2018, 6, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Saremi, M.; Sabet, F.H. Nano hydroxyapatite coating on stainless steel 304 by electrochemical method. Int. J. Mod. Phys. B 2008, 22, 3092–3098. [Google Scholar] [CrossRef]
- Suzuki, O. Evolution of Octacalcium Phosphate Biomaterials. In Octacalcium Phosphate Biomaterials; Woodhead Publishing: Sawston, UK, 2019; ISBN 9780081025116. [Google Scholar]










| Deposition Strategy | Substrate | Temperature (°C) | Time (h) | Precursor Addition | Applied Potential (V) | Specimen Code |
|---|---|---|---|---|---|---|
| 1 | Ti | 80 | 1 | Ca * | −1.5 | HA1 |
| 2 | Ti | 80 | 2 | Ca * | −1.5 | HA2 |
| 3 | Ti | 80 | 4 | Ca * | −1.5 | HA3 |
| 4 | Ti | 80 | 1 | P * | −1.5 | HA4 |
| 5 | Ti | 80 | 2 | P * | −1.5 | HA5 |
| 6 | Ti | 80 | 4 | P * | −1.5 | HA6 |
| 7 | Ti | 90 | 1 | Ca * | −1.5 | HA7 |
| 8 | Ti | 90 | 2 | Ca * | −1.5 | HA8 |
| 9 | Ti | 90 | 4 | Ca * | −1.5 | HA9 |
| 10 | Ti | 90 | 1 | P * | −1.5 | HA10 |
| 11 | Ti | 90 | 2 | P * | −1.5 | HA11 |
| 12 | Ti | 90 | 4 | P * | −1.5 | HA12 |
| 13 | Ti | 80 | 4 | Ca + P ** | −1.5 | HA13 |
| 14 | C55 | 80 | 4 | Ca + P ** | −1.5 | HA14 |
| 15 | C55 | 80 | 4 | Ca * | −1.5 | HA15 |
| 16 | Cu | 80 | 4 | Ca + P ** | −1.5 | HA16 |
| 17 | Cu | 80 | 4 | Ca * | −1.5 | HA17 |
| 18 | Ni | 80 | 4 | Ca + P ** | −1.5 | HA18 |
| 19 | Ni | 80 | 4 | Ca * | −1.5 | HA19 |
| Sample Code | HA1 | HA2 | HA3 | HA4 | HA5 | HA6 | HA7 | HA8 | HA9 | HA10 | HA11 | HA12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystallite Size, XS (nm) | 7.13 ± 0.27 | 6.95 ± 0.33 | 7.81 ± 0.16 | 6.36 ± 0.2 | 6.98 ± 0.22 | 6.81 ± 0.27 | 9.07 ± 0.17 | 9.51 ± 0.25 | 12.24 ± 0.39 | 7.44 ± 0.21 | 8.96 ± 0.28 | 6.83 ± 0.38 |
| Sample Code | HA1 | HA2 | HA3 | HA4 | HA5 | HA6 | HA7 | HA8 | HA9 | HA10 | HA11 | HA12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thickness (µm) | 126.332 ± 2.46 | 79.856 ± 0.64 | 13.136 ± 0.42 | 24.646 ± 0.11 | 66.956 ± 0.79 | 167.074 ± 3.15 | 13.809 ± 0.26 | 13.157 ± 0.06 | 64.527 ± 1.25 | 48.219 ± 1.12 | 74.757 ± 0.17 | 55.917 ± 0.58 |
| Sample Code | HA13 | Ha3 | HA14 | HA15 | HA16 | HA17 | HA18 | HA19 |
|---|---|---|---|---|---|---|---|---|
| Crystallite Size, XS (nm) | 7.54 | 7.81 | - | 8.45 | - | 8.00 | - | 9.54 |
| Sample Code | HA13 | HA3 | HA14 | HA15 | HA16 | HA17 | HA18 | HA19 |
|---|---|---|---|---|---|---|---|---|
| Thickness (µm) | 5.847 ± 0.07 | 13.136 ± 0.42 | 185.88 ± 4.89 | 35.104 ± 0.11 | 101.907 ± 7.7 | 117.136 ± 1.4 | 93.918 ± 3.32 | 45.783 ± 0.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taranu, B.-O.; Ianasi, P.; Rus, S.F.; Bucur, A.I. Simultaneous Precipitation and Electrodeposition of Hydroxyapatite Coatings at Different Temperatures on Various Metal Substrates. Coatings 2022, 12, 288. https://doi.org/10.3390/coatings12020288
Taranu B-O, Ianasi P, Rus SF, Bucur AI. Simultaneous Precipitation and Electrodeposition of Hydroxyapatite Coatings at Different Temperatures on Various Metal Substrates. Coatings. 2022; 12(2):288. https://doi.org/10.3390/coatings12020288
Chicago/Turabian StyleTaranu, Bogdan-Ovidiu, Paula Ianasi, Stefania Florina Rus, and Alexandra Ioana Bucur. 2022. "Simultaneous Precipitation and Electrodeposition of Hydroxyapatite Coatings at Different Temperatures on Various Metal Substrates" Coatings 12, no. 2: 288. https://doi.org/10.3390/coatings12020288