Preparation and Enhanced Isothermal Oxidation Resistance of a Low Diffusivity NiRePtAl Single-Phase Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coating Preparation
2.2. Oxidation Tests
2.3. Characterization
3. Results
3.1. Initial Microstructure of Coatings
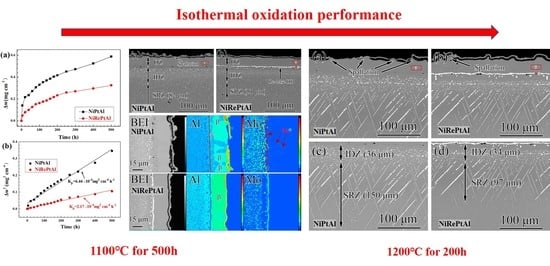
3.2. Isothermal Oxidation Behavior at 1100 °C
3.3. Isothermal Oxidation Behavior at 1200 °C
4. Discussion
4.1. The Effect of DB Layer on Inhibiting Mo Outward Diffusion
4.2. The Effect of DB Layer on Oxide Scale Growth
4.3. The Effect of BD Layer on SRZ Formation
- (a)
- During the treatment of aluminization, the γ/γ’ coherent structure was broken and transformed into β phasel; due to the diffusion fluxes of aluminum from NiPtAl coating to the Ni3Al-base matrix and that of nickel in the opposite direction. Because the solid solubility of molybdenum [30], tungsten [31] and rhenium [32] in β and γ’ is lower than in γ, and the diffusion mobility of these refractory elements is much slower than Ni, Co, Al [33], refractory elements will precipitate out as TCP phase, as shown in Figure 12a.
- (b)
- (c)
- As the interdiffusion occurs for a certain time, the chemical potential gradients of Al and Ni decreased at the IDZ growth frontier. Meanwhile, TCP precipitates dispersed in IDZ had the blocking effect of Al and Ni diffusion. Thus, the γ/γ’→β was replaced by γ/γ’→γ’. When the diffusion fluxes of Al and Ni become too low to support the interdiffusion zone growth, SRZ is formed. The β→γ/γ’ transformation at the interface of the IDZ/SRZ, and the β/γ’ interface will enter the outer coating.
5. Conclusions
- (1)
- Low diffusivity NiRePtAl single-phase coatings exhibited superior oxidation performance than NiPtAl coatings, both at 1100 and 1200 °C.
- (2)
- The outward diffusion of molybdenum was restrained due to the Re-based diffusion barrier.
- (3)
- The elemental interdiffusion was significantly restrained by the Re diffusion barrier layer, in which the thickness of secondary reaction zone and the amount of TCP phase were notably reduced.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.F.; Jiang, C.Y.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. Effect of aluminisation characteristics on the microstructure of single phase beta-(Ni,Pt)Al coating and the isothermal oxidation behaviour. Corros. Sci. 2016, 106, 43–54. [Google Scholar] [CrossRef]
- Jiang, C.; Qian, L.; Feng, M.; Liu, H.; Bao, Z.; Chen, M.; Zhu, S.; Wang, F. Benefits of Zr addition to oxidation resistance of a sin-gle-phase (Ni,Pt)Al coating at 1373 K. J. Mater. Sci. Technol. 2019, 35, 1334–1344. [Google Scholar] [CrossRef]
- Song, Y.; Murakami, H.; Zhou, C. Cyclic-Oxidation Behavior of Multilayered Pt/Ru-Modified Aluminide Coating. J. Mater. Sci. Technol. 2011, 27, 280–288. [Google Scholar] [CrossRef]
- Yu, C.T.; Liu, H.; Jiang, C.Y.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. Modification of NiCoCrAlY with Pt: Part II. Application in TBC with pure metastable tetragonal(t’) phase YSZ and thermal cycling behavior. J. Mater. Sci. Technol. 2019, 35, 350–359. [Google Scholar] [CrossRef]
- Divya, V.D.; Ramamurty, U.; Paul, A. Effect of Pt on interdiffusion and mechanical properties of the γ and γ′ phases in the Ni–Pt–Al system. Philos. Mag. 2012, 92, 2187–2214. [Google Scholar] [CrossRef]
- Paul, A.; Kodentsov, A.A.; van Loo, F.J.J. On diffusion in the β-NiAl phase. J. Alloys Compd. 2005, 403, 147–153. [Google Scholar] [CrossRef]
- Peng, X.; Jiang, S.; Gong, J.; Sun, X.; Sun, C. Preparation and Hot Corrosion Behavior of a NiCrAlY + AlNiY Composite Coating. J. Mater. Sci. Technol. 2016, 32, 587–592. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Jiang, C.Y.; Yu, C.T.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. Preparation and oxidation performance of a low-diffusion Pt-modified aluminide coating with Re-base diffusion barrier. Corros. Sci. 2020, 168, 108582. [Google Scholar] [CrossRef]
- Kiruthika, P.; Paul, A. A pseudo-binary interdiffusion study in the β-Ni(Pt)Al phase. Philos. Mag. Lett. 2015, 95, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Xu, M.; Li, S.; Bao, Z.; Zhu, S.; Wang, F. Improving cyclic oxidation resistance of Ni3Al-based single crystal superalloy with low-diffusion platinum-modified aluminide coating. J. Mater. Sci. Technol. 2020, 54, 132–143. [Google Scholar] [CrossRef]
- Yao, H.; Bao, Z.; Shen, M.; Zhu, S.; Wang, F. A magnetron sputtered microcrystalline β-NiAl coating for SC superalloys. Part II. Effects of a NiCrO diffusion barrier on oxidation behavior at 1100 °C. Appl. Surf. Sci. 2017, 407, 485–494. [Google Scholar] [CrossRef]
- Guo, C.; Wang, W.; Cheng, Y.; Zhu, S.; Wang, F. Yttria partially stabilised zirconia as diffusion barrier between NiCrAlY and Ni-base single crystal René N5 superalloy. Corros. Sci. 2015, 94, 122–128. [Google Scholar] [CrossRef]
- Peng, H.; Guo, H.; He, J.; Gong, S. Cyclic oxidation and diffusion barrier behaviors of oxides dispersed NiCoCrAlY coatings. J. Alloys Compd. 2010, 502, 411–416. [Google Scholar] [CrossRef]
- Wang, L.; Gorr, B.; Christ, H.-J.; Mukherji, D.; Rösler, J. The effect of alloyed nickel on the short-term high temperature oxidation behaviour of Co–Re–Cr-based alloys. Corros. Sci. 2015, 93, 19–26. [Google Scholar] [CrossRef]
- Tong, Y.; Bai, S.; Zhang, H.; Ye, Y. Rhenium coating prepared on carbon substrate by chemical vapor deposition. Appl. Surf. Sci. 2012, 261, 390–395. [Google Scholar] [CrossRef]
- Huang, Y.; Bai, S.; Zhang, H.; Ye, Y. Sealing of cracks in the rhenium coating on C/C composites by alloying with cobalt. Surf. Coat. Technol. 2015, 261, 404–410. [Google Scholar] [CrossRef]
- Mercier, S.; Boivin, D.; Bacos, M.P.; Josso, P. A Novel Duplex Re-NiW Based Diffusion Barrier on a Nickel-Base Superalloy for TBC Systems. Mater. Sci. Forum 2008, 595–598, 127–134. [Google Scholar] [CrossRef]
- Yu, C.T.; Liu, H.; Ullah, A.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. High-temperature performance of (Ni,Pt)Al coatings on sec-ond-generation Ni-base single-crystal superalloy at 1100 °C: Effect of excess S impurities. Corros. Sci. 2019, 159, 108115. [Google Scholar] [CrossRef]
- Barin, I. Cl-Cu2Te. In Thermochemical Data of Pure Substances; Wiley-VCH: Weinheim, Germany; New York, NY, USA; Basel, Switzerland, 1995; pp. 524–634. [Google Scholar]
- Das, D.K.; Murphy, K.S.; Ma, S.; Pollock, T.M. Formation of Secondary Reaction Zones in Diffusion Aluminide-Coated Ni-Base Single-Crystal Superalloys Containing Ruthenium. Metall. Mater. Trans. A 2008, 39, 1647–1657. [Google Scholar] [CrossRef]
- Rae, C.; Hook, M.; Reed, R. The effect of TCP morphology on the development of aluminide coated superalloys. Mater. Sci. Eng. A 2005, 396, 231–239. [Google Scholar] [CrossRef]
- Suzuki, A.S.; Rae, C.M.F. Comparison of microstructural evolution in Pt aluminised Ni based superalloys with and without Ru. Mater. Sci. Technol. 2013, 29, 726–732. [Google Scholar] [CrossRef]
- Das, D.K.; Gleeson, B.; Murphy, K.S.; Ma, S.; Pollock, T.M. Formation of secondary reaction zone in ruthenium bearing nickel based single crystal superalloys with diffusion aluminide coatings. Mater. Sci. Technol. 2009, 25, 300–308. [Google Scholar] [CrossRef]
- Murakami, H.; Sakai, T. Anisotropy of secondary reaction zone formation in aluminized Ni-based single-crystal superalloys. Scr. Mater. 2008, 59, 428–431. [Google Scholar] [CrossRef]
- Kasai, K.; Murakami, H.; Kuroda, S.; Imai, H. Effect of Surface Treatment and Crystal Orientation on Microstructural Changes in Aluminized Ni-Based Single-Crystal Superalloy. Mater. Trans. 2011, 52, 1768–1772. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Gong, X.; Peng, H.; Ma, Y.; Guo, H. Interdiffusion behavior between NiAlHf coating and Ni-based single crystal superalloy with different crystal orientations. Appl. Surf. Sci. 2014, 326, 124–130. [Google Scholar] [CrossRef]
- Kiruthika, P.; Makineni, S.; Srivastava, C.; Chattopadhyay, K.; Paul, A. Growth mechanism of the interdiffusion zone between platinum modified bond coats and single crystal superalloys. Acta Mater. 2016, 105, 438–448. [Google Scholar] [CrossRef]
- Kasai, K.; Murakami, H.; Noda, K. Effect of Thermal History on Microstructural Changes in Aluminized Nickel-Based Single-Crystal Superalloy. Mater. Trans. 2013, 54, 2252–2257. [Google Scholar] [CrossRef] [Green Version]
- Nystrom, J.D.; Pollock, T.M.; Murphy, W.H.; Garg, A. Discontinuous cellular precipitation in a high-refractory nickel-base superalloy. Metall. Mater. Trans. A 1997, 28, 2443–2452. [Google Scholar] [CrossRef]
- Havrankova, J.; Bursik, J.; Kroupa, A.; Broz, P. Experimental study and thermodynamic assessment of the Ni–Al–Cr–Mo system at 1173 K. Scripta Mater. 2001, 45, 121–126. [Google Scholar] [CrossRef]
- Kainuma, R.; Ohnuma, I.; Ishida, K. Partition of alloying elements between γ (L12), η (DO24), β (B2) and H(L21) phases in the Ni-AI-Ti base systems. J. Chim. Phys. Phys. Chim. Biol. 1997, 94, 978–985. [Google Scholar] [CrossRef]
- Huang, W.; Chang, Y.A. A thermodynamic description of the Ni–Al–Cr–Re system. Mater. Sci. Eng. A 1999, 259, 110–119. [Google Scholar] [CrossRef]
- Campbell, C.; Boettinger, W.; Kattner, U. Development of a diffusion mobility database for Ni-base superalloys. Acta Mater. 2002, 50, 775–792. [Google Scholar] [CrossRef]
- Wöllmer, S.; Zaefferer, S.; Göken, M.; Mack, T.; Glatzel, U. Characterization of phases of aluminized nickel base superalloys. Surf. Coat. Technol. 2003, 167, 83–96. [Google Scholar] [CrossRef]
- Ning, B.; Weaver, M. A preliminary study of DC magnetron sputtered NiAl–Hf coatings. Surf. Coat. Technol. 2004, 177–178, 113–120. [Google Scholar] [CrossRef]












| At.% | Al | Cr | Ni | Mo | Pt | Re |
|---|---|---|---|---|---|---|
| 1 | 30.12 | 3.42 | 60.73 | 0.91 | 4.82 | 0 |
| 2 | 33.26 | 3.15 | 58.56 | 0 | 5.03 | 0 |
| 3 | 4.24 | 14.13 | 29.24 | 30.04 | 0 | 22.35 |
| At.% | Al | Cr | Ni | Mo | Pt | Re |
|---|---|---|---|---|---|---|
| 4 | 26.42 | 2.17 | 64.25 | 2.86 | 4.30 | 0 |
| 5 | 30.93 | 1.77 | 62.99 | 0.33 | 3.98 | 0 |
| 6 | 6.13 | 10.15 | 25.87 | 32.17 | 0 | 25.68 |
| At.% | Al | Cr | Ni | Mo | Pt | Re |
|---|---|---|---|---|---|---|
| 7 | 24.18 | 4.65 | 63.86 | 3.19 | 4.12 | 0 |
| 8 | 30.36 | 2.07 | 61.81 | 1.48 | 4.28 | 0 |
| 9 | 3.26 | 8.15 | 29.23 | 36.22 | 0 | 23.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Yang, E.; Guo, C.; Liu, Q.; Yang, L.; Bao, Z. Preparation and Enhanced Isothermal Oxidation Resistance of a Low Diffusivity NiRePtAl Single-Phase Coating. Coatings 2022, 12, 114. https://doi.org/10.3390/coatings12020114
Liu H, Yang E, Guo C, Liu Q, Yang L, Bao Z. Preparation and Enhanced Isothermal Oxidation Resistance of a Low Diffusivity NiRePtAl Single-Phase Coating. Coatings. 2022; 12(2):114. https://doi.org/10.3390/coatings12020114
Chicago/Turabian StyleLiu, He, Erqi Yang, Cean Guo, Quan Liu, Lanlan Yang, and Zebin Bao. 2022. "Preparation and Enhanced Isothermal Oxidation Resistance of a Low Diffusivity NiRePtAl Single-Phase Coating" Coatings 12, no. 2: 114. https://doi.org/10.3390/coatings12020114