Plasma–Solution Junction for the Formation of Carbon Material
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Experimental Setup
2.2. Langmuir Probe Measurement and SROES Measurement
2.3. Product Characterization
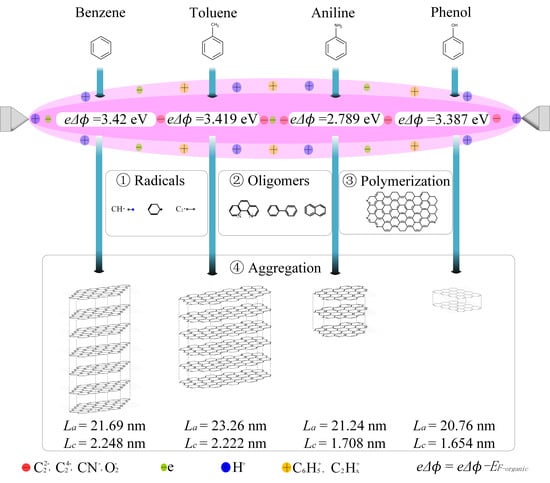
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sibul, R.; Kibena-Põldsepp, E.; Ratso, S.; Kook, M.; Sougrati, M.T.; Käärik, M.; Merisalu, M.; Aruväli, J.; Paiste, P.; Treshchalov, A.; et al. Iron- and nitrogen-doped graphene-based catalysts for fuel cell applications. Chem. ElectroChem. 2020, 7, 1739–1747. [Google Scholar]
- Han, M.; Lin, Z.; Ji, X.; Mu, Y.; Li, J.; Yu, J. Growth of flexible and porous surface layers of vertical graphene sheets for accommodating huge volume change of silicon in lithium-ion battery anodes. Mater. Today Energy 2020, 17, 100445. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K.; Bahru, R. Carbon and graphene quantum dots in fuel cell application: An overview. Int. J. Energy Res. 2020, 45, 1396–1424. [Google Scholar] [CrossRef]
- Basu, K.; Selopal, G.S.; Mohammadnezad, M.; Akilimali, R.; Wang, Z.M.; Zhao, H.; Vetrone, F.; Rosei, F. Hybrid graphene/metal oxide anodes for efficient and stable dye sensitized solar cell. Electrochim. Acta 2020, 349, 136409. [Google Scholar] [CrossRef]
- Mo, R.; Tan, X.; Li, F.; Tao, R.; Xu, J.; Kong, D.; Wang, Z.; Xu, B.; Wang, X.; Wang, C.; et al. Tin-graphene tubes as anodes for lithium-ion batteries with high volumetric and gravimetric energy densities. Nat. Commun. 2020, 11, 1374. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Qiao, Y.; Guo, R.; Naveed, S.; Hirtz, T.; Li, X.; Fu, Y.; Wei, Y.; Deng, G.; Yang, Y.; et al. Triode-mimicking graphene pressure sensor with positive resistance variation for physiology and motion monitoring. ACS Nano 2020, 14, 10104–10114. [Google Scholar] [CrossRef]
- Park, J.M.; Cao, Y.; Watanabe, K.; Taniguchi, T.; Jarillo-Herrero, P. Tunable strongly coupled superconductivity in magic-angle twisted trilayer graphene. Nature 2021, 590, 249–255. [Google Scholar] [CrossRef]
- Guo, B.; Fang, L.; Zhang, B.; Gong, J.R. Graphene doping: A review. Insci. J. 2011, 1, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Novodchuk, I.; Kayaharman, M.; Ibrahim, K.; Al-Tuairqi, S.; Irannejad, M.; Abdel-Rahman, E.; Sanderson, J.; Bajcsy, M.; Yavuz, M. B/n co-doped graphene oxide gel with extremely-high mobility and ion/ioff for large-area field effect transistors. Carbon 2020, 158, 624–630. [Google Scholar] [CrossRef]
- Ye, S.; Luo, F.; Xu, T.; Zhang, P.; Shi, H.; Qin, S.; Wu, J.; He, C.; Ouyang, X.; Zhang, Q.; et al. Boosting the alkaline hydrogen evolution of ru nanoclusters anchored on b/n–doped graphene by accelerating water dissociation. Nano Energy 2020, 68, 104301. [Google Scholar] [CrossRef]
- Zan, R.; Altuntepe, A. Nitrogen doping of graphene by cvd. J. Mol. Struct. 2020, 1199, 127026. [Google Scholar] [CrossRef]
- Li, D.; Duan, X.; Sun, H.; Kang, J.; Zhang, H.; Tade, M.O.; Wang, S. Facile synthesis of nitrogen-doped graphene via low-temperature pyrolysis: The effects of precursors and annealing ambience on metal-free catalytic oxidation. Carbon 2017, 115, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Huang, J.; Chen, X.; Wang, L.; Ye, Z. Powder metallurgy template growth of 3d n-doped graphene foam as binder-free cathode for high-performance lithium/sulfur battery. Carbon 2018, 137, 368–378. [Google Scholar] [CrossRef]
- Peyghan, A.A.; Beheshtian, J. Application of hexa-peri-hexabenzocoronene nanographene and its b, n, and bn doped forms in na-ion batteries: A density functional theory study. Thin Solid Films 2020, 704, 137979. [Google Scholar] [CrossRef]
- Yonezawa, T.; Čempel, D.; Nguyen, M.T. Microwave-induced plasma-in-liquid process for nanoparticle production. Bull. Chem. Soc. Jpn. 2018, 91, 1781–1798. [Google Scholar] [CrossRef]
- Bratescu, M.A.; Kim, K.; Saito, N. Quantitative spectrochemical analysis of solution plasma in aromatic molecules. Plasma Process. Polym. 2019, 16, e1900012. [Google Scholar] [CrossRef]
- Chae, S.; Bratescu, M.A.; Saito, N. Synthesis of few-layer graphene by peeling graphite flakes via electron exchange in solution plasma. J. Phys. Chem. C 2017, 121, 23793–23802. [Google Scholar] [CrossRef]
- Chae, S.; Panomsuwan, G.; Bratescu, M.A.; Teshima, K.; Saito, N. P-type doping of graphene with cationic nitrogen. ACS Appl. Nano Mater. 2019, 2, 1350–1355. [Google Scholar] [CrossRef]
- Heo, Y.K.; Lee, S.H.; Bratescu, M.A.; Kim, S.M.; Lee, G.J.; Saito, N. Generation of non-equilibrium condition in solution plasma discharge using low-pass filter circuit. Plasma Process. Polym. 2016, 14, 1600163. [Google Scholar] [CrossRef]
- Hyun, K.; Saito, N. The solution plasma process for heteroatom-carbon nanosheets: The role of precursors. Sci. Rep. 2017, 7, 3825. [Google Scholar] [CrossRef] [Green Version]
- Hyun, K.; Ueno, T.; Panomsuwan, G.; Li, O.L.; Saito, N. Heterocarbon nanosheets incorporating iron phthalocyanine for oxygen reduction reaction in both alkaline and acidic media. Phys. Chem. Chem. Phys. 2016, 18, 10856–10863. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.; Ueno, T.; Saito, N. Synthesis of nitrogen-containing carbon by solution plasma in aniline with high-repetition frequency discharges. Jpn. J. Appl. Phys. 2016, 55, 01AE18. [Google Scholar] [CrossRef]
- Islam, M.Z.; Watthanaphanit, A.; Chae, S.; Saito, N. Li–air battery and orr activity of nanocarbons produced with good synthesis rate by solution plasma process. Mater. Adv. 2021, 2, 2636–2641. [Google Scholar] [CrossRef]
- Jedsukontorn, T.; Ueno, T.; Saito, N.; Hunsom, M. Facile preparation of defective black tio2 through the solution plasma process: Effect of parametric changes for plasma discharge on its structural and optical properties. J. Alloy Compd. 2017, 726, 567–577. [Google Scholar] [CrossRef]
- Kim, D.W.; Li, O.L.; Saito, N. Enhancement of orr catalytic activity by multiple heteroatom-doped carbon materials. Phys. Chem. Chem. Phys 2015, 17, 407–413. [Google Scholar] [CrossRef]
- Kim, D.-w.; Li, O.L.; Pootawang, P.; Saito, N. Solution plasma synthesis process of tungsten carbide on n-doped carbon nanocomposite with enhanced catalytic orr activity and durability. RSC Adv. 2014, 4, 16813–16819. [Google Scholar] [CrossRef]
- Kim, K.; Chokradjaroen, C.; Saito, N. Solution plasma: New synthesis method of n-doped carbon dots as ultra-sensitive fluorescence detector for 2,4,6-trinitrophenol. Nano Express 2020, 1, 020043. [Google Scholar] [CrossRef]
- Lee, H.; Bratescu, M.A.; Ueno, T.; Saito, N. Solution plasma exfoliation of graphene flakes from graphite electrodes. RSC Adv. 2014, 4, 51758–51765. [Google Scholar] [CrossRef]
- Lee, H.; Ueno, T.; Saito, N. The effect of electrode gap distance on the synthesis of carbon materials by using solution plasma process. JOM 2015, 67, 2550–2556. [Google Scholar] [CrossRef]
- Lee, S.; Heo, Y.; Bratescu, M.A.; Ueno, T.; Saito, N. Solution plasma synthesis of a boron-carbon-nitrogen catalyst with a controllable bond structure. Phys. Chem. Chem. Phys. 2017, 19, 15264–15272. [Google Scholar] [CrossRef]
- Lee, S.; Saito, N. Enhancement of nitrogen self-doped nanocarbons electrocatalyst via tune-up solution plasma synthesis. RSC Adv. 2018, 8, 35503–35511. [Google Scholar] [CrossRef] [PubMed]
- Morishita, T.; Ueno, T.; Panomsuwan, G.; Hieda, J.; Yoshida, A.; Bratescu, M.A.; Saito, N. Fastest formation routes of nanocarbons in solution plasma processes. Sci. Rep. 2016, 6, 36880. [Google Scholar] [CrossRef] [Green Version]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Simple one-step synthesis of fluorine-doped carbon nanoparticles as potential alternative metal-free electrocatalysts for oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 9972–9981. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Nitrogen-doped carbon nanoparticles derived from acrylonitrile plasma for electrochemical oxygen reduction. Phys. Chem. Chem. Phys. 2015, 17, 6227–6232. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Fe–n-doped carbon-based composite as an efficient and durable electrocatalyst for the oxygen reduction reaction. RSC Adv. 2016, 6, 114553–114559. [Google Scholar] [CrossRef]
- Phan, P.Q.; Chae, S.; Pornaroontham, P.; Muta, Y.; Kim, K.; Wang, X.; Saito, N. In situ synthesis of copper nanoparticles encapsulated by nitrogen-doped graphene at room temperature via solution plasma. RSC Adv. 2020, 10, 36627–36635. [Google Scholar] [CrossRef]
- Niu, J.; Chokradjaroen, C.; Saito, N. Graphitic n-doped graphene via solution plasma with a single dielectric barrier. Carbon 2022, 199, 347–356. [Google Scholar] [CrossRef]
- Banno, M.; Akaike, K.; Yui, H. Time-resolved optical diagnostics of solution plasma formed with graphite electrodes. Jpn. J. Appl. Phys. 2018, 57, 0102B3. [Google Scholar] [CrossRef] [Green Version]
- Banno, M.; Kanno, K.; Someya, Y.; Yui, H. Nanosecond time-resolved microscopic spectroscopy for diagnostics of an atmospheric-pressure discharge plasma formed in aqueous solution. Jpn. J. Appl. Phys. 2015, 54, 066101. [Google Scholar] [CrossRef]
- Saito, N.; Bratescu, M.A.; Hashimi, K. Solution plasma: A new reaction field for nanomaterials synthesis. Jpn. J.Appl. Phys. 2018, 57, 0102A4. [Google Scholar] [CrossRef]
- Nascimento, J.C.; Aragão, E.C.B.B.; Fernandes, A.D.; Barbosa, F.T.F.; Costa, L.M.S.; Sousa, D.C.; Oliveira, C.; Abreu, G.J.P.; Grigorov, K.G.; Getsov, P.; et al. Optical measurements of an atmospheric pressure microplasma jet aiming surface treatment. Am. J. Condens. Matter Phys. 2014, 4, 8–18. [Google Scholar] [CrossRef]
- Wang, C.; An, H.H.; Xiong, J.; Fang, Z.H.; Wang, Y.W.; Zhang, Z.; Hua, N.; Sun, J.R.; Wang, W. A pinhole camera for ultrahigh-intensity laser plasma experiments. Rev. Sci. Instrum. 2017, 88, 113501. [Google Scholar] [CrossRef] [PubMed]
- Chokradjaroen, C.; Wang, X.; Niu, J.; Fan, T.; Saito, N. Fundamentals of solution plasma for advanced materials synthesis. Mater. Today Adv. 2022, 14, 100244. [Google Scholar] [CrossRef]
- Arumugam, S.; Perumal, M.; Anjana, K.P.; Satyanarayna, S.V.M.; Sinha, S.K. Plasma–metal junction. Phys. Plasmas 2020, 27, 023512. [Google Scholar] [CrossRef]
- Chen, C.; Fu, W.; Zhang, C.; Lu, D.; Han, M.; Yan, Y. Langmuir probe diagnostics with optical emission spectrometry (oes) for coaxial line microwave plasma. Appl. Sci. 2020, 10, 8117. [Google Scholar] [CrossRef]
- Hippler, R.; Cada, M.; Hubicka, Z. Time-resolved langmuir probe diagnostics of a bipolar high power impulse magnetron sputtering discharge. Appl. Phys. Lett. 2020, 116, 064101. [Google Scholar] [CrossRef]
- Smy, P.R. The use of langmuir probes in the study of high pressure plasmas. Adv. Phys. 1976, 25, 517–553. [Google Scholar] [CrossRef]
- Smy, P.R.; Noor, A.I. High-pressure langmuir probe in a weak flowing plasma or a plasma sheath. J. Appl. Phys. 1976, 47, 1327–1331. [Google Scholar] [CrossRef]
- Chen, Q.; Kaneko, T.; Hatakeyama, R. Characterization of pulse-driven gas-liquid interfacial discharge plasmas and application to synthesis of gold nanoparticle-DNA encapsulated carbon nanotubes. Curr. Appl. Phys. 2011, 11, S63–S66. [Google Scholar] [CrossRef]
- Chen, Q.; Kaneko, T.; Matsuda, N.; Hatakeyama, R. Potential structure of discharge plasma inside liquid directly measured by an electrostatic probe. Appl. Phys. Lett. 2013, 102, 244105. [Google Scholar] [CrossRef]
- Zhang, T.; Mariaux, G.; Vardelle, A.; Li, C.-J. Numerical simulation of plasma jet characteristics under very low-pressure plasma spray conditions. Coatings 2021, 11, 726. [Google Scholar] [CrossRef]
- Kiel, R.E. Continuum electrostatic probe theory for large sheaths on spheres and cylinders. J.Appl. Phys. 1969, 40, 3668–3673. [Google Scholar] [CrossRef]
- Liu, W.; Niu, J.; Zhao, S.; Chai, M. Study on atmospheric pressure glow discharge based on ac-dc coupled electric field. J. Appl. Phys. 2018, 123, 023303. [Google Scholar] [CrossRef] [Green Version]
- Qayyum, A.; Naseer, M.U.; Deeba, F.; Ahmad, S.; Shah, S.I.W.; Imran, M.; Hussain, S.; Zakaullah, M. Swept langmuir probe investigation of a time varying dc discharge. SN Appl. Sci. 2021, 3, 84. [Google Scholar] [CrossRef]
- Kaneko, T.; Chen, Q.; Harada, T.; Hatakeyama, R. Structural and reactive kinetics in gas–liquid interfacial plasmas. Plasma Sources Sci. Technol. 2011, 20, 034014. [Google Scholar] [CrossRef]
- Kaneko, T.; Harada, T.; Qiang, C.; Hatakeyama, R. Synthesis of nanoparticles conjugated with carbon nanotubes using gas-liquid interfacial plasmas. In Proceedings of the Tencon 2010–2010 IEEE Region 10 Conference, Fukuoka, Japan, 21–24 November 2010; pp. 149–153. [Google Scholar]
- Cui, W.; Liu, W.; Gao, Y. A “self-triggered” arc initiation applied in vacuum arc thrusters. Europhys. Lett. 2019, 125, 15001. [Google Scholar] [CrossRef]
- Cherrington, B.E. The use of electrostatic probes for plasma diagnostics--a review. Plasma Chem. Plasma Process. 1982, 2, 113–140. [Google Scholar] [CrossRef]
- Fanara, C.; Vilarinho, L. Electrical characterization of atmospheric pressure arc plasmas. Eur. Phys. J. D At. Mol. Opt. Phys. 2004, 28, 241–251. [Google Scholar] [CrossRef]
- Fanara, C. Sweeping electrostatic probes in atmospheric pressure arc plasmas-part i: General observations and characteristic curves. IEEE Trans. Plasma Sci. 2005, 33, 1072–1081. [Google Scholar] [CrossRef]
- Richardson, C.F.a.I.M. A langmuir multi-probe system for the characterization of atmospheric pressure arc plasmas. J. Phys. D Appl. Phys. 2001, 34, 2715–2725. [Google Scholar]
- Blair, L.; Xu, K.G. Langmuir probe diagnostics of an atmospheric-pressure microplasma. In Proceedings of the 46th AIAA Plasmadynamics and Lasers Conference, Dallas, TX, USA, 22–26 June 2015. [Google Scholar]
- Clements, R.M.; Smy, P.R. Ion current from a collision-dominated flowing plasma to a cylindrical electrode surrounded by a thin sheath. J. Appl. Phys. 1970, 41, 3745–3749. [Google Scholar] [CrossRef]
- Xu, K.G.; Doyle, S.J. Measurement of atmospheric pressure microplasma jet with langmuir probes. J. Vac. Sci. Technol. A Vac. Surf. Films 2016, 34, 051301. [Google Scholar] [CrossRef]
- Vanraes, P.; Nikiforov, A.; Leys, C. Electrical and spectroscopic characterization of underwater plasma discharge inside rising gas bubbles. J. Phys. D Appl. Phys. 2012, 45, 245206. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, Q.; Wang, Y. Semiconductor-based nanocomposites for photocatalytic H2 production and CO2 conversion. Phys. Chem. Chem. Phys. 2013, 15, 2632–2649. [Google Scholar] [CrossRef] [PubMed]
- Michael, G.; Walter, E.L.W.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar]
- van der Horst, R.M.; Verreycken, T.; van Veldhuizen, E.M.; Bruggeman, P.J. Time-resolved optical emission spectroscopy of nanosecond pulsed discharges in atmospheric-pressure N2 and N2/H2O mixtures. J. Phys. D Appl. Phys. 2012, 45, 345201. [Google Scholar] [CrossRef] [Green Version]
- Coros, M.; Varodi, C.; Pogacean, F.; Gal, E.; Pruneanu, S.M. Nitrogen-doped graphene: The influence of doping level on the charge-transfer resistance and apparent heterogeneous electron transfer rate. Sensors 2020, 20, 1815. [Google Scholar] [CrossRef] [PubMed]





| Plasma Phase X1 | Plasma–Gas Phase X2 | Gas–Liquid Phase X3 | SROES | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ne (a) | Te (eV) | ϕp (V) | Ne (a) | Te (eV) | ϕp (V) | Ne (a) | Te (eV) | ϕp (V) | Te (eV) | Ne-stark (a) | |
| Benzene | 1.99 | 3.66 | 0.12 | 3.50 | 0.56 | 0.12 | 2.98 | 0.51 | 0.08 | 0.333 | 7.64 |
| Toluene | 2.81 | 3.38 | 0.29 | 5.02 | 0.35 | 0.33 | 3.24 | 0.30 | 0.02 | 0.327 | 4.16 |
| Phenol | 2.53 | 3.18 | 0.15 | 4.76 | 0.60 | 0.55 | 3.70 | 0.50 | 0.20 | 0.335 | 4.80 |
| Aniline | 2.50 | 3.25 | 0.22 | 3.66 | 0.32 | 0.22 | 2.66 | 0.26 | 0.21 | 0.322 | 2.98 |
| Precursor | XRD | Raman | ||||||
|---|---|---|---|---|---|---|---|---|
| 2θ/θ | d/(nm) | Lc/(nm) | n | IWC-36°/IC002 | ID/IG | I2D/IG | La/(nm) | |
| Benzene | 23.66 | 0.384 | 2.248 | 5.854 | 1.850 | 0.89 | 0.34 | 21.69 |
| Toluene | 23.28 | 0.390 | 2.222 | 5.697 | 0.880 | 0.83 | 0.39 | 23.26 |
| Phenol | 24.00 | 0.379 | 1.654 | 4.364 | 0.743 | 0.93 | 0.34 | 20.76 |
| Aniline | 23.74 | 0.383 | 1.708 | 4.460 | 1.089 | 0.91 | 0.48 | 21.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, J.; Chokradjaroen, C.; Sawada, Y.; Wang, X.; Saito, N. Plasma–Solution Junction for the Formation of Carbon Material. Coatings 2022, 12, 1607. https://doi.org/10.3390/coatings12111607
Niu J, Chokradjaroen C, Sawada Y, Wang X, Saito N. Plasma–Solution Junction for the Formation of Carbon Material. Coatings. 2022; 12(11):1607. https://doi.org/10.3390/coatings12111607
Chicago/Turabian StyleNiu, Jiangqi, Chayanaphat Chokradjaroen, Yasuyuki Sawada, Xiaoyang Wang, and Nagahiro Saito. 2022. "Plasma–Solution Junction for the Formation of Carbon Material" Coatings 12, no. 11: 1607. https://doi.org/10.3390/coatings12111607