Influence of Al Doping on the Morphological, Structural and Gas Sensing Properties of Electrochemically Deposited ZnO Films on Quartz Resonators
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, R.; Ding, H.; Kim, K.; Peng, Z.; Wu, J.; Culp, J.T.; Ohodnicki, P.R.; Beckman, E.; Chen, K.P. Metal-organic framework functionalized polymer coating for fiber optical methane sensors. Sens. Actuators B 2020, 324, 128627. [Google Scholar] [CrossRef]
- Leong, A.; Saha, T.; Swamy, V.; Ramakrishnan, N. A langasite crystal microbalance coated with graphene oxide-platinum nanocomposite as a volatile organic compound sensor: Detection and discrimination characteristics. Sensors 2020, 20, 334. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Zhang, L.; Xing, H.; Bai, P.; Liu, B.; Wang, C.; Lei, K.; Wang, H.; Peng, S.; Yang, S. Gas sensing of ordered and disordered structure SiO2 and their adsorption behavior based on quartz crystal microbalance. Sens. Actuators B 2020, 305, 127479. [Google Scholar] [CrossRef]
- Cretescu, I.; Lutic, D.; Manea, L.R. Electrochemical Sensors for Monitoring of Indoor and Outdoor Air Pollution. In Electrochemical Sensors Technology; Rahman, M.M., Asiri, A.M., Eds.; IntechOpen Limited: London, UK, 2016; Chapter 4. [Google Scholar] [CrossRef] [Green Version]
- Jian, Y.; Hu, W.; Zhao, Z.; Cheng, P.; Haick, H.; Yao, M.; Wu, W. Gas sensors based on chemi-resistive hybrid functional nanomaterials. Nano-Micro Lett. 2020, 12, 71. [Google Scholar] [CrossRef] [Green Version]
- Saruhan, B.; Roussin Lontio Fomekong, R.L.; Nahirniak, S. Review: Influences of semiconductor metal oxide properties on gas sensing characteristics. Front. Sens. 2021, 2, 657931. [Google Scholar] [CrossRef]
- Chen, X.; Leishman, M.; Bagnall, D.; Nasiri, N. Nanostructured gas sensors: From air quality and environmental monitoring to healthcare and medical applications. Nanomaterials 2021, 11, 1927. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.N.; Ganesh, R.S.; Karthigeyan, A. Effect of annealing temperature on structural, optical and electrical properties of hydrothermal assisted zincoxide nanorods. Thin Solid Films 2016, 598, 39–45. [Google Scholar] [CrossRef]
- Navaneethan, M.; Archana, J.; Arivanandhan, M.; Hayakawa, Y. Functional properties of amine-passivated ZnO nanostructures and dye-sensitized solar cell characteristics. Chem. Eng. J. 2012, 213, 70–77. [Google Scholar] [CrossRef]
- Ganesh, S.R.; Durgadevi, E.; Navaneethan, M.; Patil, V.L.; Ponnusamy, S.; Muthamizhchelvan, C.; Kawasaki, S.; Patil, P.S.; Hayakawa, Y. Tuning the selectivity of NH3 gas sensing response using Cu-doped ZnO nanostructures. Sens. Actuators A 2018, 269, 331–341. [Google Scholar] [CrossRef]
- Laurenti, M.; Stassi, S.; Canavese, G.; Cauda, V. Surface Engineering of Nanostructured ZnO Surfaces. Adv. Mater. Interfaces 2016, 4, 1600758. [Google Scholar] [CrossRef]
- Bhati, V.S.; Hojamberdiev, M.; Kumar, M. Enhanced sensing performance of ZnO nanostructures-based gas sensors: A review. Energy Rep. 2020, 6, 46–62. [Google Scholar] [CrossRef]
- Ganesh, R.S.; Navaneethan, M.; Mani, G.K.; Ponnusamy, S.; Tsuchiya, K.; Muthamizhchelvan, C.; Kawasaki, S.; Hayakawa, Y. Influence of Al doping on the structural, morphological, optical, and gas sensing properties of ZnO nanorods. J. Alloys Compd. 2017, 698, 555–564. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Z.; Liu, S.; Shi, Y.; Dong, Y.; Feng, W. Maize straw-templated hierarchical porous ZnO:Ni with enhanced acetone gas sensing properties. Sens. Actuators B 2017, 243, 1224–1230. [Google Scholar] [CrossRef]
- Maswanganye, M.W.; Rammutla, K.E.; Mosuang, T.; Mwakikunga, B.W. The effect of Co and in combinational or individual doping on the structural, optical and selective sensing properties of ZnO nanoparticles. Sens. Actuators B 2017, 247, 228–237. [Google Scholar] [CrossRef]
- Hjiri, M.; El Mir, L.; Leonardi, S.G.; Pistone, A.; Mavilia, L.; Neri, G. Al-doped ZnO for highly sensitive CO gas sensors. Sens. Actuators B Chem. 2014, 196, 413–420. [Google Scholar] [CrossRef]
- Sahay, P.P.; Nath, R.K. Al-doped ZnO thin films as methanol sensors. Sens. Actuators B Chem. 2008, 134, 654–659. [Google Scholar] [CrossRef]
- Zhong, W.W.; Liu, F.M.; Cai, L.G.; Peng-Ding; Zhou, C.C.; Zeng, L.G.; Liu, X.Q.; Li, Y. Elaboration and characterization of Al doped ZnO nanorod thin films annealed in hydrogen. J. Alloys Compd. 2011, 509, 3847–3851. [Google Scholar] [CrossRef]
- Dimitrov, I.G.; Dikovska, A.O.; Atanasov, P.A.; Stoyanchov, T.R.; Vasilev, T. Al doped ZnO thin films for gas sensor application. J. Phys. Conf. Ser. 2008, 113, 012044. [Google Scholar] [CrossRef] [Green Version]
- Raj, I.L.P.; Gobalakrishnan, S.; Praseetha, P.K.; Chidhambaram, N.; Saravanakumar, S.; Ganesh, V.; AlFaify, S.; Algarni, H.; Yahia, I.S. Improved ammonia vapor sensing properties of Al-doped ZnO nanoparticles prepared by sol-gel process. Phys. Scr. 2021, 96, 085802. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, Y.L.; Gong, F.L.; Xi, K.F.; Liu, M.; Zhang, H.L.; Fang, S.M. Al doped narcissus-like ZnO for enhanced NO2 sensing performance: An experimental and DFT investigation. Sens. Actuators B Chem. 2020, 305, 127489. [Google Scholar] [CrossRef]
- Vattappalam, S.C.; Thomas, D.; Augustine, S.; Mathew, S. Effect of electron irradiation on gas sensing properties of Al-ZnO. Cogent Phys. 2015, 2, 1019664. [Google Scholar] [CrossRef]
- Lovchinov, K.; Ganchev, M.; Petrov, M.; Nichev, H.; Rachkova, A.; Angelov, O.; Mikli, V.; Dimova-Malinovska, D. Structural and optical properties of electrochemically deposited ZnO films in electrolyte containing Al2(SO4)3. Phys. Status Solidi A 2013, 210, 743–747. [Google Scholar] [CrossRef]
- Cheng, H.; Qin, L.; Li, F.; Wang, K.M. Ammonia sensing characteristics of quartz resonator coated with ZnO nanowires sensitive layer. In Proceedings of the IEEE International Frequency Control Symposium, Honolulu, HI, USA, 19–21 May 2008. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Roto, R.; Rianjanu, A.; Rahmawati, A.; Fatyadi, I.A.; Yulianto, N.; Majid, N.; Syamsu, I.; Wasisto, H.S.; Triyana, K. Quartz Crystal Microbalances Functionalized with Citric Acid-Doped Polyvinyl Acetate Nanofibers for Ammonia Sensing. ACS Appl. Nano Mater. 2020, 3, 5687–5697. [Google Scholar]
- Minh, V.A.; Tuan, L.A.; Huy, T.Q.; Hung, V.N.; Quy, N.V. Enhanced NH3 gas sensing properties of a QCM sensor by increasing the length of vertically orientated ZnO nanorods. Appl. Surf. Sci. 2013, 265, 458–464. [Google Scholar] [CrossRef]
- Andre, R.S.; Kwak, D.; Dong, Q.; Zhong, W.; Correa, D.S.; Mattoso, L.H.C.; Lei, Y. Sensitive and selective NH3 monitoring at room temperature using zno ceramic nanofibers decorated with poly(styrene sulfonate). Sensors 2018, 18, 1058. [Google Scholar] [CrossRef] [Green Version]
- Si, P.; Mortensen, J.; Komolov, A.; Denborg, J.; Møller, P.J. Polymer coated quartz crystal microbalance sensors for detection of volatile organic compounds in gas mixtures. Anal. Chim. Acta 2007, 597, 223–230. [Google Scholar] [CrossRef]
- Tu, Y.; Kyle, C.; Luo, H.; Zhang, D.; Das, A.; Briscoe, J.; Dunn, S.; Titirici, M.; Krause, S. Ammonia gas sensor response of a vertical zinc oxide nanorod-gold junction diode at room temperature. ACS Sens. 2020, 5, 3568–3575. [Google Scholar] [CrossRef]
- Zheng, Z.Q.; Yao, J.D.; Wang, B.; Yang, G.W. Light-controlling, flexible and transparent ethanol gas sensor based on ZnO nanoparticles for wearable devices. Sci. Rep. 2015, 5, 11070. [Google Scholar] [CrossRef] [PubMed]
- Strashilov, V.L.; Alexieva, G.E.; Velichkov, V.N.; Mateva, R.P.; Avramov, I.D. Polymer-coated quartz microbalance sensors for volatile organic compound gases. Sens. Lett. 2009, 7, 203–211. [Google Scholar] [CrossRef]
- Devi, K.; Selvan, G.; Karunakaran, M.; Raj, I.; El-Rehim, A.; Zahran, H.; Shkir, M.; AlFaify, S. Room temperature ammonia gas sensing properties of Al-doped ZnO nanostructured thin films. Opt. Quantum Electron. 2020, 52, 501. [Google Scholar] [CrossRef]
- Kathwate, L.H.; Umadevi, G.; Kulal, P.M.; Nagaraju, P.; Duba, D.P.; Nanjundan, A.K.; Mote, V.D. Ammonia Gas sensing properties of Al doped ZnO thin films. Sens. Actuators A Phys. 2020, 313, 112193. [Google Scholar] [CrossRef]
- Anasthasiya, A.N.A.; Ramya, S.; Balamurugan, D.; Rai, P.K.; Jeyaprakash, B.G. Adsorption property of volatile molecules on ZnO nanowires: Computational and experimental approach. Bull. Mater. Sci. 2018, 41, 4. [Google Scholar] [CrossRef] [Green Version]





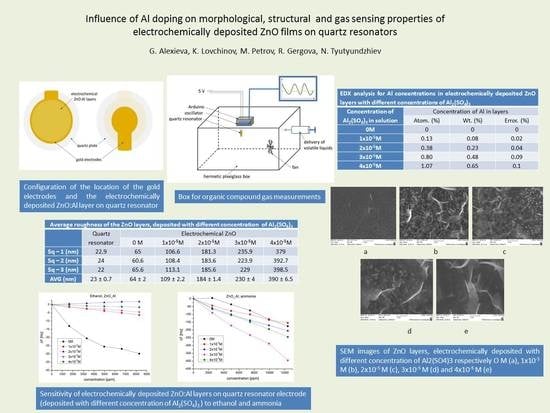
| - | Quartz Resonator | Electrochemical ZnO | ||||
|---|---|---|---|---|---|---|
| 0 M | 1 × 10−5 M | 2 × 10−5 M | 3 × 10−5 M | 4 × 10−5 M | ||
| Sq–1 (nm) | 22.9 | 65 | 106.6 | 181.3 | 235.9 | 379 |
| Sq–2 (nm) | 24 | 60.6 | 108.4 | 183.6 | 223.9 | 392.7 |
| Sq–3 (nm) | 22 | 65.6 | 113.1 | 185.6 | 229 | 398.5 |
| AVG (nm) | 23 ± 0.7 | 64 ± 2 | 109 ± 2.2 | 184 ± 1.4 | 230 ± 4 | 390 ± 6.5 |
| Concentration of Al2(SO4)3 in Solution | Concentration of Al in Layers | ||
|---|---|---|---|
| Atom. (%) | Wt. (%) | Error. (%) | |
| 0 M | 0 | 0 | 0 |
| 1 × 10−5 M | 0.13 | 0.08 | 0.02 |
| 2 × 10−5 M | 0.38 | 0.23 | 0.04 |
| 3 × 10−5 M | 0.80 | 0.48 | 0.09 |
| 4 × 10−5 M | 1.07 | 0.65 | 0.1 |
| Conc. of Al2(SO4)3 | F0, Hz | Δf, Hz | Δm, g |
|---|---|---|---|
| 0 M | 10,007,464 | 5020 | 7.12 × 10−6 |
| 1 × 10−5 M | 10,005,805 | 15,037 | 2.13 × 10−5 |
| 2 × 10−5 M | 10,008,952 | 13,276 | 1.88 × 10−5 |
| 3 × 10−5 M | 10,007,835 | 12,700 | 1.81 × 10−5 |
| 3 × 10−5 M | 10,005,734 | 12,932 | 1.84 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexieva, G.; Lovchinov, K.; Petrov, M.; Gergova, R.; Tyutyundzhiev, N. Influence of Al Doping on the Morphological, Structural and Gas Sensing Properties of Electrochemically Deposited ZnO Films on Quartz Resonators. Coatings 2022, 12, 81. https://doi.org/10.3390/coatings12010081
Alexieva G, Lovchinov K, Petrov M, Gergova R, Tyutyundzhiev N. Influence of Al Doping on the Morphological, Structural and Gas Sensing Properties of Electrochemically Deposited ZnO Films on Quartz Resonators. Coatings. 2022; 12(1):81. https://doi.org/10.3390/coatings12010081
Chicago/Turabian StyleAlexieva, Gergana, Konstantin Lovchinov, Miroslav Petrov, Rositsa Gergova, and Nikolay Tyutyundzhiev. 2022. "Influence of Al Doping on the Morphological, Structural and Gas Sensing Properties of Electrochemically Deposited ZnO Films on Quartz Resonators" Coatings 12, no. 1: 81. https://doi.org/10.3390/coatings12010081